Regulatory Support for Drug Approvals
Regulatory support for drug approvals is a pivotal factor impacting the chorea market in Japan. The Pharmaceuticals and Medical Devices Agency (PMDA) has streamlined the approval process for new therapies, particularly for rare diseases like chorea. This regulatory environment encourages pharmaceutical companies to invest in research and development, as the pathway to market entry becomes more efficient. In recent years, the PMDA has implemented initiatives to expedite the review of innovative treatments, which may lead to a quicker availability of new therapies for patients. As a result, the chorea market is likely to experience growth driven by the introduction of novel drugs that address unmet medical needs.
Technological Innovations in Diagnostics
Technological advancements in diagnostic tools are transforming the landscape of the chorea market in Japan. Innovations such as genetic testing and advanced imaging techniques enable earlier and more accurate diagnosis of chorea-related disorders. For instance, the introduction of next-generation sequencing has improved the identification of genetic mutations associated with Huntington's disease, facilitating timely intervention. The Japanese healthcare system is increasingly adopting these technologies, which may lead to a higher diagnosis rate and, consequently, a growing patient population requiring treatment. This trend suggests that as diagnostic capabilities improve, the chorea market could experience substantial growth, driven by the need for targeted therapies and personalized medicine.
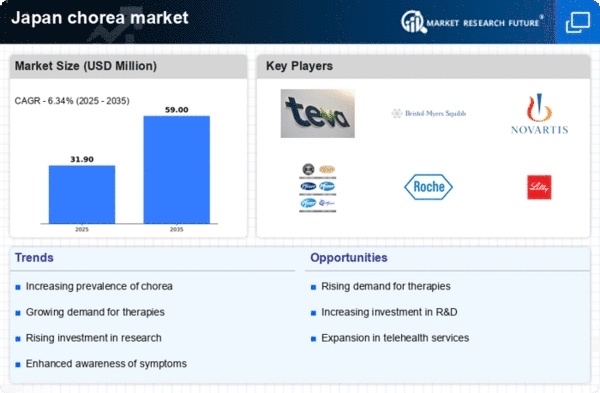
Increasing Prevalence of Chorea Disorders
The rising incidence of chorea disorders in Japan appears to be a significant driver for the chorea market. Recent studies indicate that the prevalence of Huntington's disease, a primary cause of chorea, affects approximately 5.7 individuals per 100,000 in the Japanese population. This growing number of affected individuals necessitates enhanced healthcare services and treatment options, thereby expanding the market. Furthermore, the aging population in Japan, which is projected to reach 36.2% by 2040, may contribute to an increase in neurodegenerative disorders, including chorea. As the population ages, the demand for effective therapies and management strategies in the chorea market is likely to rise, prompting pharmaceutical companies to invest in research and development to address these needs.
Rising Investment in Neurological Research
Investment in neurological research is a crucial driver for the chorea market in Japan. The government and private sectors are increasingly allocating funds to explore new treatment modalities for neurodegenerative diseases, including chorea. In 2025, it is estimated that Japan will invest approximately ¥1 trillion in neurological research, reflecting a commitment to addressing the challenges posed by these disorders. This influx of funding is likely to accelerate the development of innovative therapies and improve patient outcomes. As research progresses, the chorea market may benefit from the introduction of novel drugs and treatment strategies, enhancing the overall landscape for patients and healthcare providers.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups in Japan is influencing the chorea market positively. These organizations play a vital role in raising awareness about chorea disorders and advocating for better healthcare policies. They provide essential resources and support for patients and families affected by these conditions, fostering a community that encourages dialogue and education. As these groups gain traction, they may drive demand for more comprehensive treatment options and increased funding for research. The presence of active advocacy groups can lead to improved healthcare access and resources, ultimately benefiting the chorea market by promoting awareness and encouraging innovation.