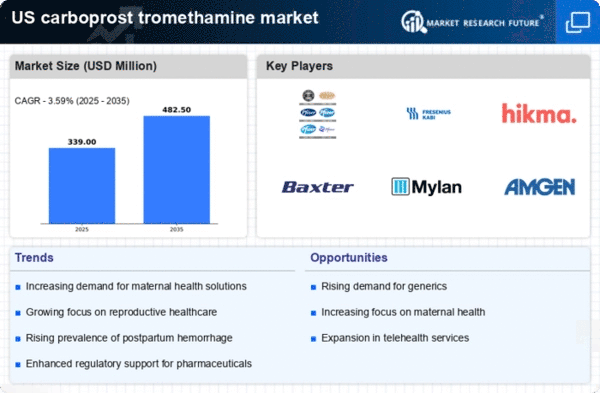
Growing Awareness of Uterine Atony
The carboprost tromethamine market is positively impacted by the growing awareness of uterine atony among healthcare providers and patients. Uterine atony, a condition characterized by the failure of the uterus to contract effectively after childbirth, is a leading cause of postpartum hemorrhage. Educational campaigns and training programs are increasingly focusing on recognizing and managing this condition. As awareness increases, healthcare professionals are more likely to utilize carboprost tromethamine as a treatment option. This trend suggests a potential increase in market demand, as timely intervention with effective medications can significantly improve maternal health outcomes.
Advancements in Clinical Guidelines
The carboprost tromethamine market is influenced by the continuous evolution of clinical guidelines surrounding obstetric care. Recent updates from authoritative bodies emphasize the importance of timely intervention in cases of severe postpartum hemorrhage. As healthcare professionals adopt these updated guidelines, the utilization of carboprost tromethamine is expected to increase. The American College of Obstetricians and Gynecologists (ACOG) recommends the use of uterotonics, including carboprost, as first-line therapy for managing uterine atony. This endorsement is likely to enhance the market's growth trajectory, as adherence to clinical guidelines translates into higher adoption rates of effective treatments, thereby solidifying carboprost tromethamine's position in the market.
Increasing Demand for Postpartum Care
The carboprost tromethamine market experiences a notable surge in demand due to the increasing focus on postpartum care. Healthcare providers are recognizing the importance of managing postpartum hemorrhage effectively, which is a leading cause of maternal morbidity. In the US, the incidence of postpartum hemorrhage is estimated to affect approximately 1 in 20 deliveries, prompting a greater need for effective treatment options. Carboprost tromethamine, known for its efficacy in controlling uterine atony, is becoming a preferred choice among clinicians. This heightened awareness and demand for postpartum care solutions are likely to drive growth in the carboprost tromethamine market, as hospitals and healthcare facilities prioritize maternal health initiatives.
Rising Investment in Maternal Health Programs
Investment in maternal health programs is a significant driver for the carboprost tromethamine market. Government and private sector initiatives aimed at improving maternal health outcomes are gaining momentum. In the US, funding for maternal health initiatives has increased, with allocations reaching over $500 million annually. These investments often focus on enhancing access to essential medications, including carboprost tromethamine, which is critical for managing complications during and after childbirth. As healthcare systems prioritize maternal health, the demand for effective treatments like carboprost tromethamine is likely to rise, contributing to the overall growth of the market.
Technological Innovations in Drug Delivery Systems
Technological advancements in drug delivery systems are poised to influence the carboprost tromethamine market positively. Innovations such as pre-filled syringes and smart delivery devices enhance the ease of administration and improve patient compliance. These advancements are particularly relevant in emergency settings, where timely intervention is crucial for managing postpartum hemorrhage. The introduction of user-friendly delivery systems may lead to increased adoption of carboprost tromethamine in clinical practice. As healthcare providers seek efficient solutions for urgent maternal health needs, the market for carboprost tromethamine is likely to expand, driven by these technological innovations.