Cost-Effectiveness of Montelukast
The montelukast api market benefits from the cost-effectiveness of montelukast as a treatment option for asthma and allergic rhinitis. Compared to other therapeutic alternatives, montelukast is often more affordable, making it accessible to a broader patient population. The NHS has been focusing on cost containment strategies, which include promoting the use of generic medications. As montelukast is available in generic forms, it is likely to be a preferred choice for healthcare providers and patients alike. This cost advantage may lead to increased prescriptions and, consequently, a positive impact on the montelukast api market. Furthermore, the economic burden of untreated asthma and allergies on the healthcare system underscores the importance of effective and affordable treatment options, further solidifying montelukast's position in the market.
Growing Awareness of Preventive Healthcare
There is a notable shift towards preventive healthcare in the UK, which is positively influencing the montelukast api market. Patients and healthcare providers are increasingly recognizing the importance of early intervention in managing chronic conditions such as asthma. Montelukast, being a preventive treatment, aligns well with this trend. Educational campaigns by health organizations and the NHS are raising awareness about the benefits of using montelukast for long-term asthma control. This growing emphasis on preventive measures is likely to enhance the market for montelukast, as more patients seek proactive solutions to manage their respiratory health. The integration of montelukast into asthma management plans may become more prevalent, further driving its demand in the montelukast api market.
Regulatory Support for Generic Medications
the montelukast api market is influenced by regulatory support for generic medications in the UK.. The Medicines and Healthcare products Regulatory Agency (MHRA) has streamlined the approval process for generics, facilitating quicker market entry. This regulatory environment encourages pharmaceutical companies to produce montelukast in its generic form, which is often more affordable for patients. As a result, the availability of generic montelukast is likely to increase, leading to greater market penetration. The emphasis on generic medications aligns with the NHS's objectives to reduce healthcare costs while maintaining treatment efficacy. Consequently, the montelukast api market may see a surge in demand as healthcare providers and patients opt for cost-effective treatment options.
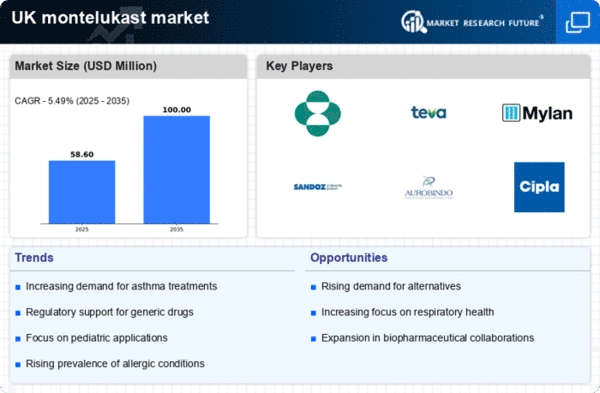
Increasing Prevalence of Asthma and Allergies
The montelukast api market is experiencing growth due to the rising prevalence of asthma and allergic conditions in the UK. Recent studies indicate that approximately 8.5% of adults and 12% of children in the UK are diagnosed with asthma, leading to a heightened demand for effective treatments. Montelukast, as a leukotriene receptor antagonist, plays a crucial role in managing these conditions. The increasing awareness of asthma management and the importance of adherence to treatment regimens further drive the montelukast api market. Additionally, the National Health Service (NHS) has been promoting the use of effective asthma medications, which may contribute to the market's expansion. As the population ages and environmental factors contribute to respiratory issues, the demand for montelukast is likely to continue its upward trajectory.
Technological Advancements in Drug Formulation
Technological advancements in drug formulation are playing a pivotal role in shaping the montelukast api market. Innovations in drug delivery systems, such as improved oral formulations and inhalation devices, enhance the efficacy and patient compliance of montelukast. These advancements may lead to better therapeutic outcomes, thereby increasing the attractiveness of montelukast as a treatment option. Furthermore, research into novel formulations that improve bioavailability and reduce side effects could expand the market potential for montelukast. As pharmaceutical companies invest in research and development to leverage these technologies, the montelukast api market is likely to benefit from enhanced product offerings and increased patient satisfaction.