Rising Incidence of Syphilis
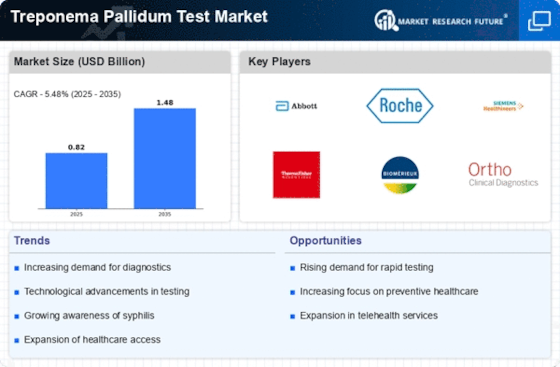
The increasing incidence of syphilis worldwide is a primary driver for the Treponema Pallidum Test Market. Reports indicate that syphilis rates have been on the rise, particularly among certain demographics, such as men who have sex with men and pregnant women. This trend necessitates enhanced testing capabilities to ensure timely diagnosis and treatment. As healthcare providers seek to address this public health concern, the demand for accurate and efficient Treponema Pallidum tests is likely to grow. The World Health Organization has highlighted the need for improved testing strategies, which may further stimulate market growth. Consequently, the Treponema Pallidum Test Market is poised to expand as healthcare systems adapt to these rising infection rates.
Increased Public Health Initiatives
Public health initiatives aimed at combating sexually transmitted infections are driving the Treponema Pallidum Test Market. Governments and non-governmental organizations are implementing awareness campaigns and screening programs to promote early detection and treatment of syphilis. These initiatives often include free or subsidized testing, which encourages individuals to seek testing services. As a result, the demand for Treponema Pallidum tests is likely to rise, as more people become aware of their sexual health and the importance of regular testing. The collaboration between public health entities and healthcare providers is essential in expanding testing access, thereby positively impacting the Treponema Pallidum Test Market.
Technological Innovations in Testing
Technological advancements in diagnostic testing are significantly influencing the Treponema Pallidum Test Market. Innovations such as point-of-care testing and rapid diagnostic tests are enhancing the speed and accuracy of syphilis detection. These technologies allow for immediate results, which is crucial for effective patient management and treatment. The integration of molecular techniques, such as PCR, is also gaining traction, providing higher sensitivity and specificity. As healthcare facilities increasingly adopt these advanced testing methods, the Treponema Pallidum Test Market is expected to experience substantial growth. Furthermore, the development of user-friendly devices may facilitate broader access to testing, particularly in resource-limited settings.
Regulatory Support for Testing Standards
Regulatory bodies are increasingly emphasizing the importance of standardized testing protocols for sexually transmitted infections, including syphilis. This regulatory support is a significant driver for the Treponema Pallidum Test Market. Guidelines established by health authorities ensure that testing methods meet specific quality and safety standards, which can enhance public trust in testing services. Compliance with these regulations may also encourage manufacturers to innovate and improve their testing products. As healthcare providers align with these standards, the demand for reliable Treponema Pallidum tests is expected to grow, further propelling the market forward. The establishment of clear testing guidelines is likely to foster a more robust testing environment.
Growing Focus on Maternal and Child Health
The emphasis on maternal and child health is increasingly influencing the Treponema Pallidum Test Market. Pregnant women are at a higher risk for syphilis, which can have severe consequences for both the mother and the child if left untreated. As healthcare systems prioritize maternal health, the demand for routine syphilis screening during pregnancy is likely to increase. This focus is supported by various health organizations advocating for comprehensive prenatal care that includes syphilis testing. Consequently, the Treponema Pallidum Test Market is expected to benefit from this heightened awareness and the implementation of testing protocols aimed at protecting maternal and child health.