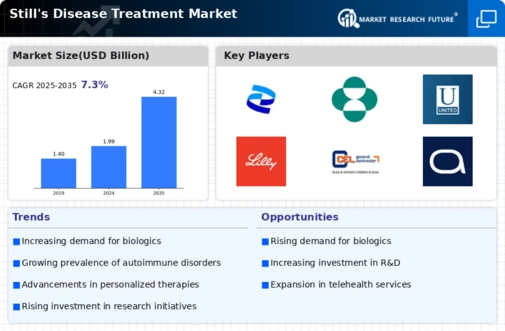
Advancements in Biologic Therapies
The emergence of biologic therapies represents a transformative shift in the Still's Disease Treatment Market. These therapies, which target specific components of the immune system, have shown promising results in managing Still's disease symptoms. Recent data indicates that biologics can significantly reduce inflammation and improve patient outcomes. As more clinical trials validate the efficacy of these treatments, healthcare providers are increasingly adopting them as first-line options. This trend not only enhances treatment efficacy but also drives market expansion, as patients and clinicians alike seek out the latest advancements in biologic therapies to address the complexities of Still's disease.
Rising Prevalence of Still's Disease
The increasing incidence of Still's disease is a notable driver for the Still's Disease Treatment Market. Recent estimates suggest that the prevalence of this rare autoimmune condition is on the rise, with more cases being diagnosed annually. This trend may be attributed to heightened awareness among healthcare professionals and advancements in diagnostic techniques. As the number of patients grows, the demand for effective treatment options intensifies, prompting pharmaceutical companies to invest in research and development. Consequently, this surge in prevalence is likely to stimulate market growth, as healthcare providers seek innovative therapies to manage the symptoms and improve the quality of life for affected individuals.
Growing Awareness and Education Initiatives
The rise in awareness and education initiatives surrounding Still's disease is influencing the treatment market positively. Healthcare organizations and advocacy groups are actively working to educate both medical professionals and the public about the condition. This increased awareness is crucial, as it leads to earlier diagnosis and treatment, which can significantly improve patient outcomes. As more individuals become informed about Still's disease, the demand for effective treatment options is expected to rise. Consequently, this heightened awareness is likely to play a pivotal role in shaping the Still's Disease Treatment Market, as it encourages healthcare providers to prioritize research and therapeutic advancements.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a vital driver in the Still's Disease Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for treatments targeting rare diseases, including Still's disease. This supportive environment encourages pharmaceutical companies to develop and bring new therapies to market more swiftly. Recent regulatory changes have streamlined the approval process for biologics and other novel treatments, which may lead to a more diverse range of options for patients. As a result, this regulatory landscape is likely to foster innovation and enhance the availability of effective treatments, thereby propelling the growth of the Still's Disease Treatment Market.
Increased Investment in Research and Development
Investment in research and development is a critical driver for the Still's Disease Treatment Market. Pharmaceutical companies are allocating substantial resources to explore novel treatment modalities and improve existing therapies. This focus on R&D is fueled by the need for more effective and targeted treatments for Still's disease, which remains a challenging condition to manage. Recent reports indicate that the global pharmaceutical R&D expenditure has reached unprecedented levels, with a significant portion directed towards autoimmune diseases. This influx of funding is likely to accelerate the development of innovative therapies, thereby enhancing the treatment landscape for Still's disease and contributing to market growth.