Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development of innovative therapies within the Splenomegaly Therapeutics Market. Initiatives aimed at expediting the approval process for new drugs are encouraging pharmaceutical companies to invest in research and development. This regulatory environment is conducive to the introduction of novel therapies that address the needs of patients suffering from splenomegaly. Recent changes in regulatory frameworks have facilitated faster access to treatments, particularly for rare diseases and conditions associated with splenomegaly. As a result, the market is likely to see a rise in the number of approved therapies, which could enhance competition and drive down costs for patients. This supportive regulatory landscape is expected to play a crucial role in shaping the future of the splenomegaly therapeutics market.
Rising Incidence of Hematological Disorders
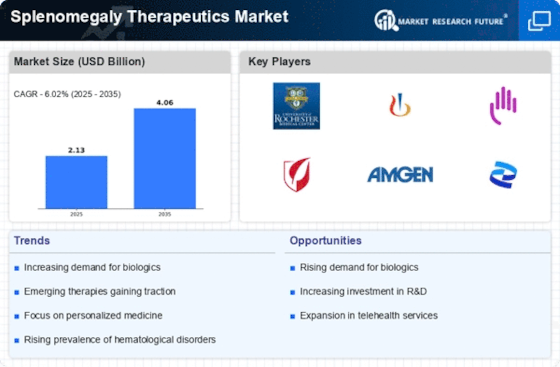
The increasing prevalence of hematological disorders, such as leukemia and lymphoma, is a primary driver for the Splenomegaly Therapeutics Market. As these conditions often lead to splenomegaly, the demand for effective therapeutic options is likely to rise. According to recent data, hematological malignancies account for a significant proportion of cancer cases, with an estimated 200,000 new cases reported annually. This trend suggests a growing patient population requiring targeted therapies, thereby propelling the splenomegaly therapeutics sector. Furthermore, advancements in diagnostic techniques have improved the identification of splenomegaly, leading to earlier intervention and treatment. Consequently, pharmaceutical companies are increasingly focusing on developing innovative therapies to address this unmet medical need, which is expected to further stimulate market growth.
Growing Awareness and Education on Splenomegaly
There is a notable increase in awareness and education regarding splenomegaly and its associated conditions, which is positively influencing the Splenomegaly Therapeutics Market. Healthcare professionals and patients are becoming more informed about the implications of splenomegaly, leading to higher rates of diagnosis and treatment. Educational initiatives and campaigns aimed at both medical practitioners and the general public are contributing to this trend. As awareness grows, patients are more likely to seek medical advice and treatment options, thereby increasing the demand for therapeutic interventions. This heightened awareness is expected to drive market growth, as more individuals are diagnosed and treated for splenomegaly-related conditions. Consequently, pharmaceutical companies are likely to respond by expanding their product offerings to meet the needs of this emerging patient population.
Advancements in Biologics and Targeted Therapies
The emergence of biologics and targeted therapies represents a transformative shift in the Splenomegaly Therapeutics Market. These innovative treatment modalities offer enhanced efficacy and reduced side effects compared to traditional therapies. The market for biologics is projected to grow substantially, with estimates suggesting a compound annual growth rate of over 10% in the coming years. This growth is driven by the increasing understanding of disease mechanisms and the development of therapies that specifically target the underlying causes of splenomegaly. As more biologics receive regulatory approval, healthcare providers are likely to adopt these therapies, leading to a more competitive landscape. Additionally, the integration of personalized medicine approaches may further enhance treatment outcomes, thereby attracting investment and research into this segment of the market.
Increased Investment in Research and Development
The Splenomegaly Therapeutics Market is experiencing a surge in investment directed towards research and development. Pharmaceutical companies are allocating substantial resources to discover and develop new therapeutic agents aimed at treating splenomegaly and its underlying causes. This trend is supported by the recognition of splenomegaly as a significant clinical issue, prompting a focus on innovative treatment solutions. Recent reports indicate that R&D spending in the pharmaceutical sector has reached unprecedented levels, with billions invested annually. This influx of funding is likely to accelerate the pace of drug development, leading to the introduction of novel therapies into the market. As a result, the therapeutic landscape for splenomegaly is expected to evolve, providing patients with more effective treatment options and enhancing overall market growth.