Aging Population
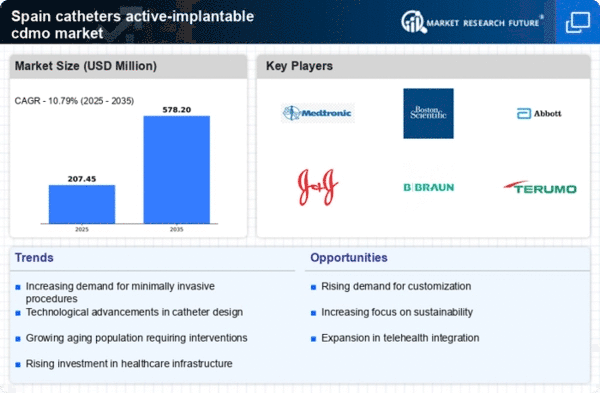
The demographic shift towards an aging population in Spain is a crucial factor impacting the catheters active-implantable-cdmo market. By 2030, it is projected that over 20% of the Spanish population will be aged 65 and older. This demographic is more susceptible to various health issues, including those requiring catheterization. As older adults often face multiple health challenges, the demand for effective and reliable catheter solutions is likely to increase. This trend suggests that manufacturers in the catheters active-implantable-cdmo market must focus on developing products that cater specifically to the needs of this demographic, potentially leading to enhanced patient care and improved health outcomes.
Investment in Healthcare Infrastructure
Spain's commitment to improving healthcare infrastructure significantly influences the catheters active-implantable-cdmo market. The government has allocated substantial funding to modernize hospitals and healthcare facilities, enhancing their capacity to provide advanced medical services. This investment is expected to reach €2 billion by 2026, facilitating the adoption of cutting-edge medical technologies, including catheters. As healthcare facilities upgrade their equipment, the demand for high-quality catheters is anticipated to rise. Consequently, this driver not only supports the growth of the catheters active-implantable-cdmo market but also encourages innovation among manufacturers to meet the evolving needs of healthcare providers.
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in Spain is a pivotal driver for the catheters active-implantable-cdmo market. Conditions such as cardiovascular diseases, diabetes, and renal disorders necessitate advanced medical interventions, including catheterization. According to recent health statistics, approximately 30% of the Spanish population suffers from at least one chronic condition, which propels the demand for effective treatment options. This trend indicates a growing need for innovative catheter solutions, as healthcare providers seek to enhance patient outcomes. the catheters active-implantable-cdmo market is likely to benefit from this increasing prevalence, as manufacturers focus on developing specialized catheters to effectively manage these chronic conditions.
Focus on Patient-Centric Healthcare Solutions
The shift towards patient-centric healthcare solutions is reshaping the landscape of the catheters active-implantable-cdmo market. Healthcare providers in Spain are increasingly prioritizing patient comfort and satisfaction, leading to a demand for catheters that minimize discomfort and enhance usability. This focus on patient experience is prompting manufacturers to innovate and develop catheters that are not only effective but also user-friendly. As a result, the market is likely to see a rise in the introduction of products designed with the patient's needs in mind. This trend indicates a potential for growth in the catheters active-implantable-cdmo market as patient-centric solutions become a standard expectation in healthcare.
Rising Awareness of Advanced Medical Technologies
There is a growing awareness among healthcare professionals and patients regarding the benefits of advanced medical technologies in Spain. This awareness is driving the adoption of innovative catheter solutions, which are perceived to enhance treatment efficacy and patient comfort. Educational initiatives and training programs are being implemented to inform healthcare providers about the latest advancements in catheter technology. As a result, the catheters active-implantable-cdmo market is likely to experience increased demand as healthcare professionals become more knowledgeable about the advantages of using advanced catheters in clinical practice. This trend may lead to a shift in treatment paradigms, favoring the use of innovative catheter solutions.