Growing Prevalence of Chronic Diseases
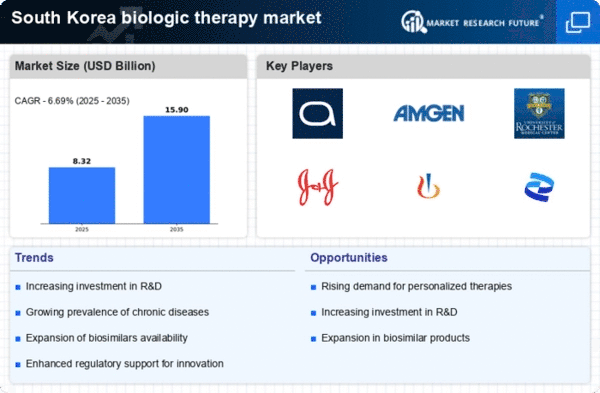
The increasing incidence of chronic diseases in South Korea is a pivotal driver for the biologic therapy market. Conditions such as rheumatoid arthritis, psoriasis, and various cancers are becoming more prevalent, necessitating advanced treatment options. According to recent health statistics, chronic diseases account for approximately 80% of healthcare expenditures in the country. This trend underscores the urgent need for effective biologic therapies, which are often more targeted and have fewer side effects compared to traditional treatments. As the population ages and lifestyle-related diseases rise, the biologic therapy market is likely to expand significantly, with a projected growth rate of around 10% annually over the next five years. This growth reflects the increasing reliance on biologics to manage complex health conditions, thereby driving innovation and investment in the sector.
Increasing Patient Awareness and Education
Patient awareness and education regarding biologic therapies are on the rise in South Korea, significantly impacting the biologic therapy market. As patients become more informed about their treatment options, there is a growing demand for advanced therapies that offer better efficacy and safety profiles. Educational campaigns by healthcare providers and patient advocacy groups have played a crucial role in disseminating information about the benefits of biologics. This heightened awareness is reflected in patient inquiries and discussions with healthcare professionals, leading to an increase in prescriptions for biologic therapies. The trend suggests that as patients become more proactive in managing their health, the biologic therapy market may see a corresponding increase in demand, potentially driving market growth by an estimated 8% annually.
Rising Healthcare Expenditure and Investment
The upward trend in healthcare expenditure in South Korea is a significant driver for the biologic therapy market. With healthcare spending projected to reach 12% of GDP by 2026, there is a growing emphasis on innovative treatment options that can improve patient outcomes. Biologic therapies, known for their efficacy in treating complex diseases, are increasingly being integrated into treatment protocols. The government and private sectors are investing heavily in healthcare infrastructure, with an estimated $2 billion allocated for the development of advanced medical technologies. This investment is likely to enhance the accessibility and affordability of biologic therapies, thereby expanding the market. As healthcare providers seek to adopt cutting-edge treatments, the biologic therapy market is expected to experience robust growth in the coming years.
Advancements in Biologic Research and Development
Innovations in research and development are propelling the biologic therapy market forward in South Korea. The country is home to numerous biotech firms and research institutions that are at the forefront of developing novel biologic therapies. Recent investments in biotechnology have led to breakthroughs in monoclonal antibodies and gene therapies, which are transforming treatment paradigms. The South Korean government has allocated substantial funding, estimated at over $500 million annually, to support biotech research initiatives. This commitment fosters a conducive environment for clinical trials and product development, enhancing the competitiveness of the biologic therapy market. As new therapies emerge, they are expected to address unmet medical needs, further stimulating market growth and attracting both domestic and international investments.
Regulatory Support and Streamlined Approval Processes
Regulatory frameworks in South Korea are increasingly supportive of the biologic therapy market, facilitating faster approval processes for new treatments. The Ministry of Food and Drug Safety (MFDS) has implemented measures to expedite the review of biologics, which is crucial for bringing innovative therapies to market. Recent reforms have reduced the average approval time for biologics to approximately 12 months, compared to 18 months previously. This efficiency not only benefits patients by providing quicker access to new therapies but also encourages pharmaceutical companies to invest in the development of biologics. The favorable regulatory environment is likely to enhance the attractiveness of the biologic therapy market, potentially leading to a surge in new product launches and increased competition among manufacturers.