Supportive Regulatory Frameworks
Supportive regulatory frameworks are emerging as a key driver in the Sequencing Kits Market. Governments and regulatory bodies are increasingly recognizing the importance of genomics in healthcare and research, leading to the establishment of guidelines that facilitate the development and approval of sequencing technologies. These frameworks not only promote innovation but also ensure the safety and efficacy of sequencing kits. As regulatory processes become more streamlined, companies are likely to invest more in research and development, further propelling the Sequencing Kits Market. This supportive environment is essential for fostering advancements in sequencing technologies and expanding their applications across various sectors.
Rising Demand for Genomic Research
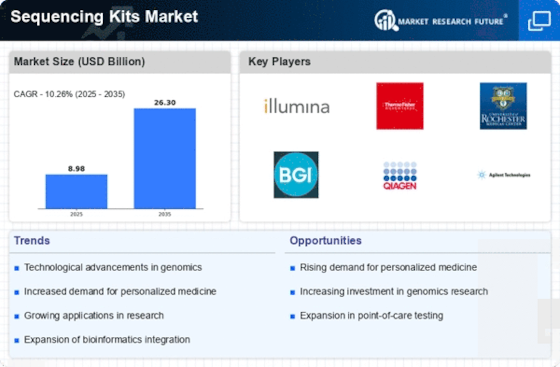
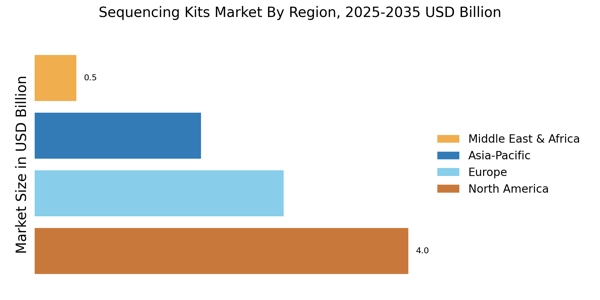
The Sequencing Kits Market is experiencing a notable surge in demand driven by the increasing focus on genomic research. As researchers and institutions strive to understand genetic variations and their implications on health, the need for high-quality sequencing kits becomes paramount. In recent years, the market has seen a compound annual growth rate (CAGR) of approximately 12%, reflecting the growing investment in genomics. This trend is further supported by the expansion of research initiatives aimed at unraveling complex diseases, which necessitate advanced sequencing technologies. Consequently, the Sequencing Kits Market is poised for continued growth as more entities recognize the value of genomic insights in both clinical and research settings.
Integration of Next-Generation Sequencing
The integration of next-generation sequencing (NGS) technologies is a pivotal driver in the Sequencing Kits Market. NGS offers unparalleled throughput and accuracy, enabling researchers to conduct large-scale genomic studies efficiently. The adoption of NGS has led to a significant reduction in sequencing costs, making it accessible to a broader range of laboratories and institutions. As of 2025, the market for NGS is projected to reach USD 10 billion, underscoring its transformative impact on the sequencing landscape. This shift towards NGS not only enhances the capabilities of researchers but also fosters innovation in personalized medicine, thereby propelling the Sequencing Kits Market forward.
Growing Applications in Clinical Diagnostics
The application of sequencing kits in clinical diagnostics is rapidly expanding, serving as a crucial driver for the Sequencing Kits Market. With the increasing prevalence of genetic disorders and the need for precise diagnostics, healthcare providers are increasingly turning to sequencing technologies. The market for clinical genomics is expected to grow significantly, with estimates suggesting it could reach USD 5 billion by 2026. This growth is attributed to the rising demand for targeted therapies and the need for comprehensive genetic testing. As healthcare systems recognize the importance of accurate diagnostics, the Sequencing Kits Market is likely to benefit from this trend, leading to enhanced patient outcomes.
Increased Collaboration Between Academia and Industry
The collaboration between academic institutions and industry players is significantly influencing the Sequencing Kits Market. Such partnerships are fostering innovation and accelerating the development of new sequencing technologies. By combining academic research with industry expertise, these collaborations are leading to the creation of cutting-edge sequencing kits that meet the evolving needs of researchers and clinicians. As of 2025, it is estimated that collaborative projects could account for over 30% of new product developments in the sequencing sector. This synergy not only enhances the capabilities of sequencing kits but also drives the overall growth of the Sequencing Kits Market, as new solutions emerge to address complex scientific challenges.