Advancements in Genetic Research
Advancements in genetic research are transforming the Schaaf Yang Syndrome Treatment Market. The identification of specific genetic mutations associated with the syndrome has opened new avenues for targeted therapies. Recent breakthroughs in gene therapy and CRISPR technology hold promise for developing effective treatments that address the root causes of the disorder. As research progresses, the potential for personalized medicine becomes increasingly viable, allowing for tailored interventions that could improve patient outcomes. Moreover, the collaboration between academic institutions and pharmaceutical companies is likely to accelerate the pace of innovation in this field. This synergy may lead to the introduction of novel therapies that not only alleviate symptoms but also modify the underlying genetic factors contributing to the syndrome, thereby reshaping the treatment landscape.
Growing Demand for Multidisciplinary Care
The Schaaf Yang Syndrome Treatment Market is increasingly influenced by the demand for multidisciplinary care approaches. Patients with Schaaf Yang Syndrome often present with a range of symptoms that require the expertise of various healthcare professionals. This complexity necessitates a collaborative approach to treatment, involving geneticists, neurologists, and behavioral specialists. As healthcare systems evolve, there is a growing recognition of the importance of integrated care models that address the diverse needs of patients. This trend is likely to enhance patient satisfaction and improve health outcomes, as coordinated care can lead to more comprehensive treatment plans. Furthermore, the emphasis on multidisciplinary care is expected to drive the development of specialized treatment centers, thereby expanding access to care for individuals affected by Schaaf Yang Syndrome.
Rising Prevalence of Schaaf Yang Syndrome
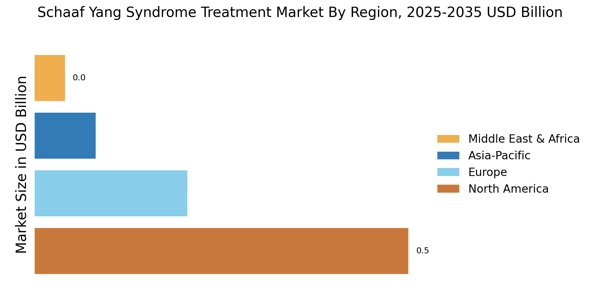
The increasing incidence of Schaaf Yang Syndrome is a pivotal driver for the Schaaf Yang Syndrome Treatment Market. As awareness of genetic disorders expands, more cases are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that the prevalence of Schaaf Yang Syndrome may be underreported, with many cases remaining undiagnosed. This growing recognition is likely to stimulate research and development efforts, thereby enhancing the treatment landscape. Furthermore, the need for specialized care and tailored therapies is becoming more pronounced, as healthcare providers seek to address the unique challenges posed by this syndrome. Consequently, the rising prevalence is expected to significantly influence market dynamics, driving investments in innovative treatment modalities and comprehensive care solutions.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a key driver in the Schaaf Yang Syndrome Treatment Market. Regulatory agencies are increasingly adopting frameworks that facilitate the expedited review and approval of treatments for rare diseases. This shift is particularly relevant for conditions like Schaaf Yang Syndrome, where traditional pathways may not adequately address the urgent need for effective therapies. Recent initiatives aimed at streamlining the approval process for orphan drugs are likely to encourage pharmaceutical companies to invest in research and development. As a result, the regulatory landscape is becoming more conducive to the introduction of novel therapies, which could significantly enhance treatment options for patients. This supportive environment is expected to foster innovation and ultimately improve the quality of care available for individuals with Schaaf Yang Syndrome.
Increased Investment in Rare Disease Research
The Schaaf Yang Syndrome Treatment Market is witnessing a surge in investment focused on rare disease research. Governments and private entities are recognizing the need for dedicated funding to address the challenges associated with rare genetic disorders. This influx of capital is facilitating the development of new therapies and enhancing clinical trial opportunities for Schaaf Yang Syndrome. Recent data indicates that funding for rare disease research has increased significantly, with many organizations prioritizing conditions that have historically been overlooked. This trend is likely to foster innovation and expedite the approval process for new treatments, ultimately benefiting patients and healthcare providers alike. As a result, the increased investment in rare disease research is expected to play a crucial role in advancing the treatment options available for Schaaf Yang Syndrome.