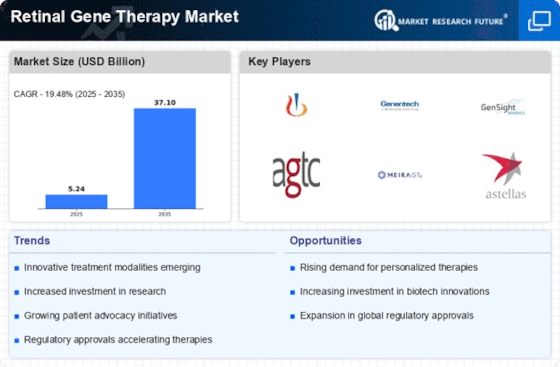
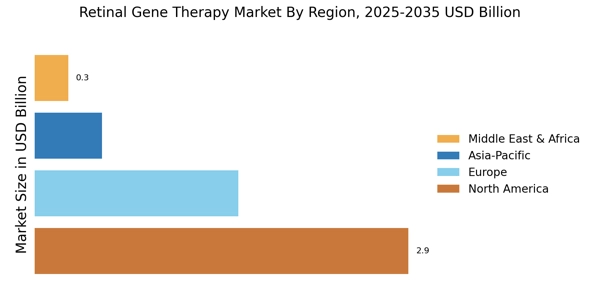Regulatory Framework Enhancements
The evolving regulatory framework surrounding gene therapies is playing a crucial role in shaping the Retinal Gene Therapy Market. Regulatory agencies are increasingly recognizing the need for expedited pathways for the approval of innovative therapies that address unmet medical needs. This shift is likely to facilitate faster access to novel treatments for patients suffering from retinal disorders. Additionally, the establishment of clear guidelines for clinical trials and post-marketing surveillance is expected to enhance the overall safety and efficacy of gene therapies. As regulatory bodies continue to adapt to the rapid advancements in biotechnology, the Retinal Gene Therapy Market is poised for growth, as companies can navigate the approval process more efficiently and bring their products to market more swiftly.
Growing Investment in Biotechnology
The surge in investment within the biotechnology sector is significantly influencing the Retinal Gene Therapy Market. Venture capital funding and public-private partnerships are increasingly directed towards companies focused on gene therapy solutions for retinal diseases. This influx of capital is facilitating the acceleration of research and development efforts, enabling the rapid advancement of innovative therapies. Furthermore, the financial backing allows for extensive clinical trials, which are essential for demonstrating the safety and efficacy of new treatments. As more companies enter the market, competition is likely to intensify, leading to a broader array of treatment options for patients. This trend underscores the potential for substantial growth within the Retinal Gene Therapy Market as investment continues to flow into this promising field.
Rising Awareness and Patient Advocacy
The increasing awareness of retinal diseases and the role of gene therapy in their treatment is driving the Retinal Gene Therapy Market. Patient advocacy groups are playing a pivotal role in educating the public and healthcare professionals about the potential benefits of gene therapies. This heightened awareness is likely to lead to greater demand for innovative treatment options, as patients seek out therapies that can address the root causes of their conditions. Furthermore, as more success stories emerge from clinical trials, the perception of gene therapy is expected to improve, encouraging more patients to explore these options. The collaboration between advocacy groups and researchers is fostering a supportive environment for the development of new therapies, thereby contributing to the growth of the Retinal Gene Therapy Market.
Advancements in Gene Editing Technologies
Recent advancements in gene editing technologies, particularly CRISPR and other genome-editing tools, are transforming the landscape of the Retinal Gene Therapy Market. These technologies enable precise modifications to the genetic material, offering the potential to correct mutations responsible for retinal diseases. The ability to target specific genes with high accuracy is likely to enhance the efficacy of gene therapies, making them more appealing to both clinicians and patients. As these technologies continue to evolve, they may lead to the development of novel therapies that can address previously untreatable conditions. The integration of these cutting-edge techniques into clinical practice is expected to drive growth in the Retinal Gene Therapy Market, as stakeholders recognize the potential for improved therapeutic outcomes.
Increasing Prevalence of Retinal Disorders
The rising incidence of retinal disorders, such as age-related macular degeneration and retinitis pigmentosa, is a primary driver for the Retinal Gene Therapy Market. It is estimated that millions of individuals are affected by these conditions, leading to a growing demand for innovative treatment options. As the population ages, the prevalence of these disorders is likely to increase, thereby propelling the market forward. The need for effective therapies that can address the underlying genetic causes of these diseases is becoming more urgent. Consequently, this trend is expected to stimulate investment in research and development, fostering advancements in gene therapy technologies. The Retinal Gene Therapy Market is thus positioned to expand significantly as healthcare providers seek to offer solutions that can improve patient outcomes and quality of life.