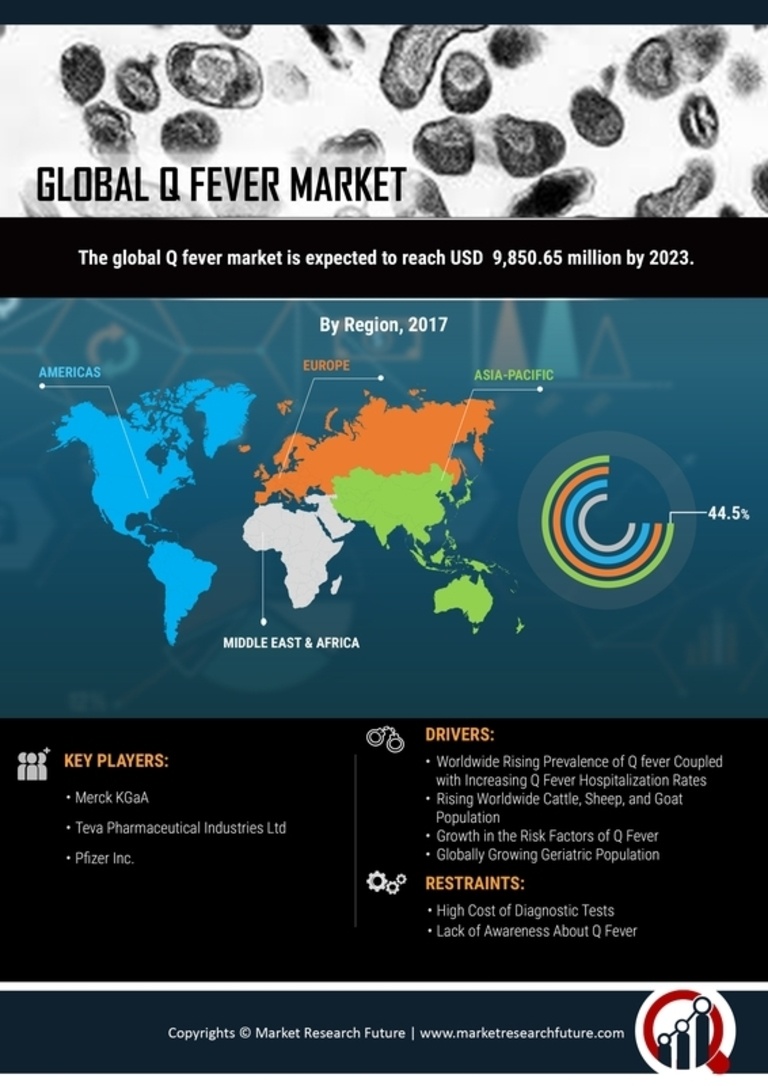
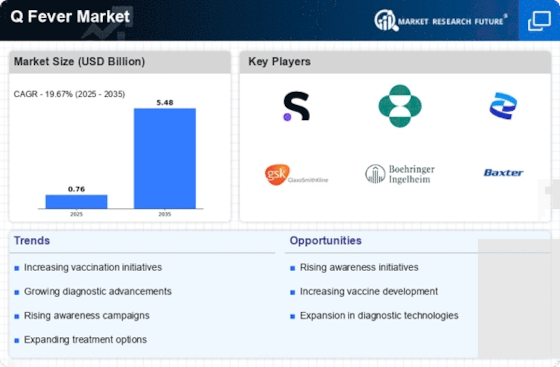
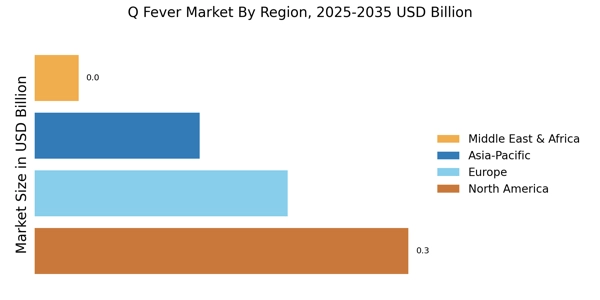
Rising Incidence of Q Fever Cases
The increasing incidence of Q Fever Market cases is a notable driver in the Q Fever Market. Recent data indicates that the number of reported cases has been on the rise, particularly in regions with high livestock populations. This trend is likely to spur demand for diagnostic tests and vaccines, as healthcare providers seek to manage and control outbreaks effectively. The heightened awareness surrounding zoonotic diseases, including Q Fever Market, has led to increased funding for research and development in this area. Consequently, the Q Fever Market is expected to experience growth as stakeholders respond to the urgent need for effective prevention and treatment options.
Growing Demand for Veterinary Vaccines
The growing demand for veterinary vaccines is a significant driver in the Q Fever Market. As livestock farming continues to expand, the need for effective vaccination programs to protect animal health becomes increasingly apparent. Vaccines that prevent Q Fever Market in livestock not only safeguard animal populations but also reduce the risk of transmission to humans. This interconnection between animal and human health underscores the importance of veterinary vaccines in controlling Q Fever Market outbreaks. Consequently, the Q Fever Market is likely to see a rise in investments aimed at developing and distributing veterinary vaccines.
Technological Innovations in Diagnostics
Technological innovations in diagnostic methods are transforming the Q Fever Market. The advent of advanced molecular techniques, such as PCR and serological assays, has significantly improved the accuracy and speed of Q Fever Market diagnosis. These innovations enable healthcare providers to identify cases more efficiently, thereby facilitating timely treatment and containment of outbreaks. As diagnostic technologies continue to evolve, the Q Fever Market is likely to witness increased adoption of these methods, leading to enhanced disease management and control strategies.
Regulatory Support for Vaccine Development
Regulatory bodies are increasingly supporting the development of vaccines against Q Fever Market, which serves as a significant driver in the Q Fever Market. Recent initiatives have streamlined the approval processes for vaccine candidates, encouraging pharmaceutical companies to invest in research and development. This regulatory support is crucial, as it not only accelerates the availability of vaccines but also enhances public confidence in vaccination programs. As a result, the Q Fever Market is poised for expansion, with a growing number of vaccine candidates entering clinical trials and potentially reaching the market in the near future.
Increased Investment in Public Health Initiatives
Increased investment in public health initiatives aimed at controlling zoonotic diseases is a critical driver in the Q Fever Market. Governments and non-governmental organizations are allocating resources to enhance surveillance, education, and vaccination programs. This focus on preventive healthcare is essential for reducing the burden of Q Fever Market and other zoonotic diseases. As funding for these initiatives grows, the Q Fever Market is expected to benefit from improved public health infrastructure and increased awareness among healthcare professionals and the general population.