Advancements in Genetic Research
Recent advancements in genetic research have significantly impacted the Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market. The identification of specific genetic mutations associated with PFIC Type 2 has opened new avenues for targeted therapies. For instance, the discovery of mutations in the ABCB11 gene has led to the development of gene therapies that may offer curative potential. As research continues to unveil the genetic underpinnings of this disorder, the market is likely to see an influx of novel treatment options tailored to individual genetic profiles. This shift towards precision medicine not only enhances treatment efficacy but also aligns with the growing trend of personalized healthcare, which is becoming increasingly important in the management of rare diseases.
Increasing Prevalence of Liver Diseases
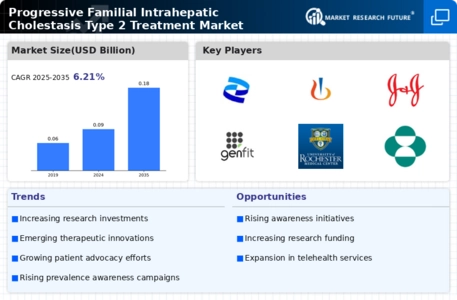
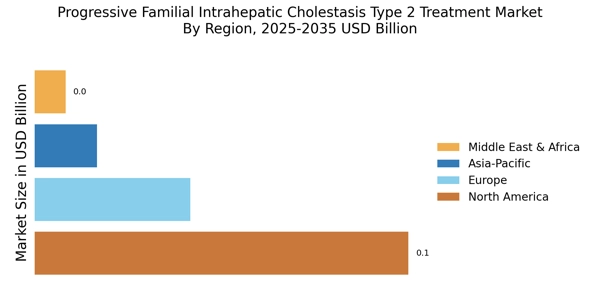
The rising incidence of liver diseases, particularly cholestatic disorders, is a primary driver for the Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market. As awareness of liver health increases, more individuals are being diagnosed with conditions like PFIC Type 2. Recent estimates suggest that the prevalence of PFIC may be around 1 in 50,000 live births, indicating a substantial patient population that requires effective treatment options. This growing patient base is likely to stimulate demand for innovative therapies and interventions, thereby propelling market growth. Furthermore, as healthcare systems evolve to address these needs, investments in research and development are expected to rise, enhancing the treatment landscape for PFIC Type 2.
Growing Investment in Rare Disease Research
The increasing investment in research and development for rare diseases is a significant driver for the Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market. Pharmaceutical companies and biotech firms are recognizing the potential profitability of developing treatments for orphan diseases, including PFIC Type 2. In recent years, funding for rare disease research has surged, with estimates indicating that the market for orphan drugs could reach over 200 billion by 2025. This influx of capital is likely to accelerate the development of innovative therapies, enhancing the treatment options available for patients suffering from PFIC Type 2. As more stakeholders enter this space, competition may also drive down costs, making treatments more accessible.
Rising Awareness and Advocacy for Rare Diseases
The growing awareness and advocacy for rare diseases are significantly influencing the Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market. Patient advocacy groups and non-profit organizations are actively working to raise awareness about PFIC Type 2, which has led to increased visibility and understanding of the condition among healthcare professionals and the general public. This heightened awareness is likely to result in earlier diagnosis and treatment, thereby expanding the patient population seeking care. Additionally, advocacy efforts are often accompanied by fundraising initiatives that support research and development, further driving innovation in treatment options. As awareness continues to grow, it is anticipated that the market for PFIC Type 2 treatments will expand, benefiting both patients and healthcare providers.
Regulatory Incentives for Orphan Drug Development
Regulatory incentives aimed at promoting the development of orphan drugs are playing a crucial role in shaping the Progressive Familial Intrahepatic Cholestasis Type 2 Treatment Market. Governments and regulatory bodies are implementing policies that provide financial benefits, such as tax credits and extended market exclusivity, to encourage pharmaceutical companies to invest in treatments for rare diseases. These incentives not only lower the financial barriers associated with drug development but also foster a more favorable environment for innovation. As a result, the number of clinical trials and new drug applications for PFIC Type 2 is expected to increase, ultimately leading to a broader array of treatment options for patients.
















Leave a Comment