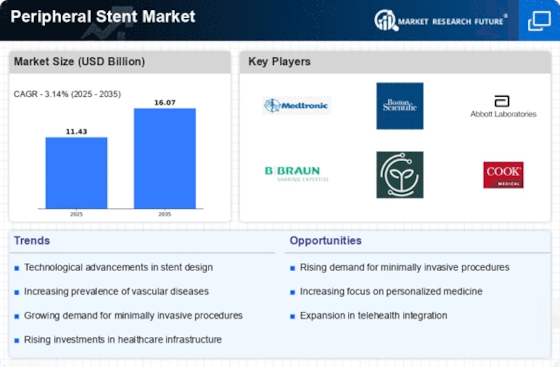
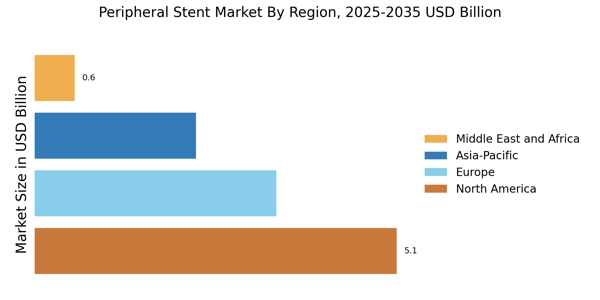
Increasing Healthcare Expenditure
Rising healthcare expenditure across various regions is a crucial driver for the Peripheral Stent Market. As governments and private sectors allocate more resources to healthcare, the availability of advanced medical technologies, including peripheral stents, is improving. This increase in funding facilitates the adoption of innovative stenting solutions, which are essential for treating conditions like PAD. Recent data indicates that healthcare spending is projected to grow at an annual rate of approximately 5% through 2025, thereby enhancing access to advanced medical treatments. This trend is likely to bolster the Peripheral Stent Market, as healthcare providers invest in state-of-the-art technologies to meet the growing demand for effective treatment options.
Regulatory Support and Approval Processes
Regulatory support and streamlined approval processes are vital factors influencing the Peripheral Stent Market. Regulatory bodies are increasingly recognizing the importance of innovative stenting technologies and are working to expedite the approval of new devices. This supportive environment encourages manufacturers to invest in research and development, leading to the introduction of advanced stent designs that meet evolving patient needs. Recent initiatives aimed at reducing the time required for clinical trials and approvals could potentially enhance market entry for new products. As a result, the Peripheral Stent Market is likely to benefit from a more dynamic and competitive landscape, fostering innovation and improving patient care.
Technological Innovations in Stent Design
Technological advancements in stent design are significantly influencing the Peripheral Stent Market. Innovations such as drug-eluting stents, bioresorbable stents, and advanced materials are enhancing the efficacy and safety of stenting procedures. These innovations not only improve patient outcomes but also reduce the risk of restenosis, a common complication associated with traditional stents. The market has witnessed a surge in the adoption of these advanced stents, with estimates indicating that the drug-eluting stent segment alone could account for over 60% of the market share by 2025. As healthcare providers increasingly favor these cutting-edge solutions, the Peripheral Stent Market is likely to experience robust growth driven by technological progress.
Rising Incidence of Peripheral Artery Disease
The increasing prevalence of peripheral artery disease (PAD) is a primary driver for the Peripheral Stent Market. As populations age, the incidence of PAD, which affects millions worldwide, continues to rise. This condition often leads to severe complications, necessitating effective treatment options. The Peripheral Stent Market is poised to benefit from this trend, as stenting procedures are commonly employed to restore blood flow in affected arteries. Recent estimates suggest that the number of PAD cases could reach over 200 million by 2025, thereby amplifying the demand for peripheral stents. This growing patient population underscores the need for innovative stent technologies that can enhance patient outcomes and reduce the risk of complications, further propelling market growth.
Growing Preference for Minimally Invasive Procedures
The shift towards minimally invasive procedures is a significant factor driving the Peripheral Stent Market. Patients and healthcare providers alike are increasingly favoring these techniques due to their associated benefits, such as reduced recovery times, lower risk of complications, and minimal scarring. As a result, the demand for peripheral stenting procedures is on the rise, with projections indicating that the market could expand at a compound annual growth rate of over 8% through 2025. This trend is further supported by advancements in imaging technologies and catheter-based techniques, which enhance the precision and effectiveness of stenting procedures. Consequently, the Peripheral Stent Market is well-positioned to capitalize on this growing preference for less invasive treatment options.