Expansion of E-commerce Platforms
The expansion of e-commerce platforms is reshaping the distribution landscape for the PEG 3350 Market. With the increasing reliance on online shopping, consumers are finding it more convenient to purchase PEG 3350 Market products from the comfort of their homes. This shift has been accelerated by the proliferation of health and wellness websites that offer a variety of PEG 3350 Market formulations. Market data indicates that online sales of PEG 3350 Market products have increased by over 20% in the past year, highlighting the growing trend of digital commerce. As e-commerce continues to thrive, the PEG 3350 Market is likely to benefit from enhanced accessibility and consumer reach.
Rising Awareness of Digestive Health
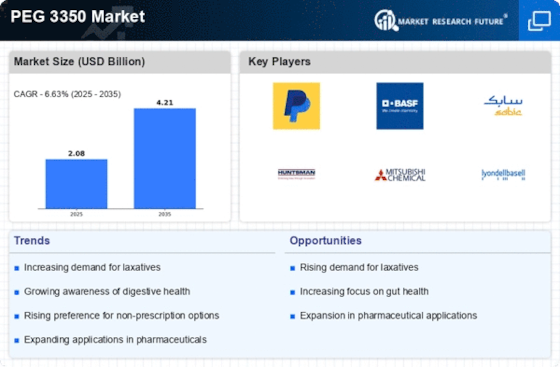
The heightened awareness surrounding digestive health is a significant driver for the PEG 3350 Market. Educational campaigns and health initiatives have led to increased public knowledge about the importance of maintaining regular bowel movements. As a result, consumers are more inclined to seek out effective solutions for constipation, with PEG 3350 Market emerging as a preferred choice due to its proven effectiveness. Market analysis indicates that the demand for PEG 3350 Market products has surged, with a reported growth rate of 10% annually. This trend suggests that as awareness continues to rise, the PEG 3350 Market will likely expand further, catering to a more informed consumer base.
Increasing Prevalence of Constipation
The rising incidence of constipation across various demographics appears to be a primary driver for the PEG 3350 Market. Studies indicate that approximately 20% of the population experiences constipation at some point, leading to a heightened demand for effective laxatives. PEG 3350 Market, known for its osmotic properties, is increasingly recommended by healthcare professionals due to its efficacy and safety profile. This trend is particularly evident among older adults, who are more susceptible to gastrointestinal issues. As awareness of digestive health continues to grow, the PEG 3350 Market is likely to see sustained growth, driven by both consumer demand and healthcare recommendations.
Technological Advancements in Formulation
Technological advancements in the formulation of PEG 3350 Market products are contributing to the growth of the PEG 3350 Market. Innovations in product delivery systems and enhanced formulations are making PEG 3350 Market more accessible and effective for consumers. For instance, the development of flavored formulations and convenient packaging options has broadened the appeal of PEG 3350 Market products, particularly among children and those who may be reluctant to use traditional laxatives. This evolution in product offerings is expected to attract a wider audience, thereby driving sales and market penetration. As these advancements continue, the PEG 3350 Market is poised for further growth.
Growing Interest in Non-Pharmaceutical Solutions
There is a notable shift towards non-pharmaceutical solutions for managing constipation, which significantly influences the PEG 3350 Market. Consumers are increasingly seeking alternatives to traditional medications, favoring products perceived as safer and more natural. PEG 3350 Market, being a polyethylene glycol-based compound, aligns with this trend as it is often viewed as a gentler option. Market data suggests that the sales of PEG 3350 Market products have increased by approximately 15% over the past year, reflecting this consumer preference. This growing interest in non-pharmaceutical solutions is expected to bolster the PEG 3350 Market, as more individuals opt for effective yet mild laxatives.