Growing Awareness and Advocacy Efforts
Growing awareness and advocacy efforts surrounding Osteogenesis Imperfecta are pivotal in driving the Osteogenesis Imperfecta Treatment Market. Increased educational initiatives and support from patient advocacy groups are helping to disseminate information about OI, its symptoms, and available treatment options. This heightened awareness is leading to earlier diagnoses and improved access to care for affected individuals. Furthermore, advocacy efforts are encouraging collaboration among researchers, healthcare providers, and pharmaceutical companies, fostering an environment conducive to innovation. As awareness continues to expand, the Osteogenesis Imperfecta Treatment Market is likely to experience increased demand for effective therapies and comprehensive care solutions.
Rising Demand for Personalized Medicine
The rising demand for personalized medicine is shaping the Osteogenesis Imperfecta Treatment Market. Patients and healthcare providers are increasingly seeking tailored treatment plans that consider individual genetic profiles and specific disease manifestations. This shift towards personalized approaches is prompting pharmaceutical companies to invest in the development of targeted therapies that can address the unique needs of OI patients. As a result, the market is likely to see a proliferation of customized treatment options, which may improve patient adherence and outcomes. The emphasis on personalized medicine is expected to drive growth in the Osteogenesis Imperfecta Treatment Market as stakeholders strive to meet the evolving expectations of patients.
Rising Prevalence of Osteogenesis Imperfecta
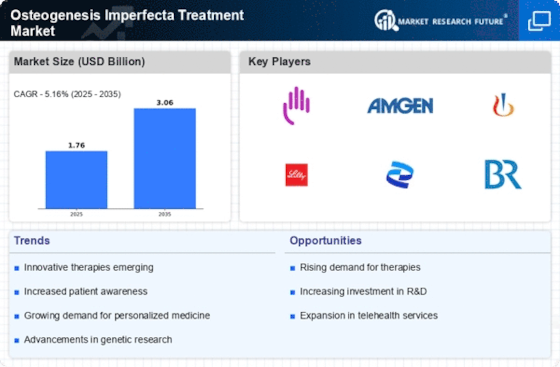
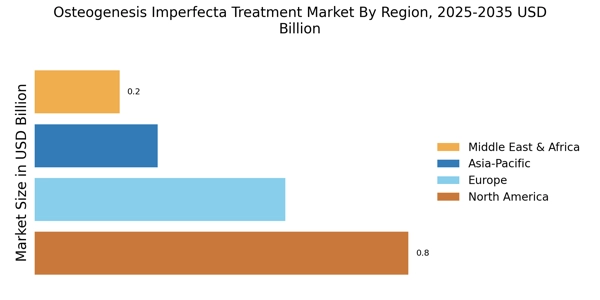
The increasing prevalence of Osteogenesis Imperfecta (OI) is a primary driver for the Osteogenesis Imperfecta Treatment Market. Recent estimates suggest that OI affects approximately 1 in 15,000 live births, leading to a growing patient population requiring effective treatment options. This rising incidence necessitates advancements in therapeutic approaches, including pharmacological treatments and surgical interventions. As awareness of OI improves, more individuals are diagnosed, further contributing to the demand for specialized care. The Osteogenesis Imperfecta Treatment Market is thus poised for growth as healthcare providers seek to address the needs of this expanding demographic, ensuring that innovative therapies are developed and made accessible.
Increased Investment in Rare Disease Research
The growing investment in rare disease research is a crucial driver for the Osteogenesis Imperfecta Treatment Market. Governments and private organizations are increasingly recognizing the need to allocate resources towards understanding and treating rare conditions like OI. This trend is reflected in the rising number of clinical trials and research initiatives focused on OI therapies. For instance, funding for research into gene editing technologies and regenerative medicine is expanding, which may lead to breakthroughs in treatment options. As more financial resources are directed towards OI research, the Osteogenesis Imperfecta Treatment Market is likely to benefit from enhanced innovation and the introduction of new therapies.
Technological Advancements in Treatment Modalities
Technological advancements in treatment modalities are significantly influencing the Osteogenesis Imperfecta Treatment Market. Innovations such as bisphosphonates, which have been shown to improve bone density and reduce fracture rates, are becoming increasingly prevalent. Additionally, the development of gene therapies and novel pharmacological agents holds promise for more effective management of OI. The market is witnessing a surge in research and development activities aimed at creating targeted therapies that address the underlying genetic causes of the disorder. As these technologies evolve, they are likely to enhance treatment outcomes and patient quality of life, thereby driving growth in the Osteogenesis Imperfecta Treatment Market.