Supportive Regulatory Environment
A supportive regulatory environment is essential for the growth of the Orthopedic Regenerative Surgical Product Market. Regulatory bodies are increasingly recognizing the potential of regenerative medicine and are streamlining approval processes for innovative products. This trend is evident in the expedited pathways for regenerative therapies, which facilitate quicker access to the market. As a result, companies are more inclined to invest in the development of new orthopedic regenerative products, knowing that regulatory hurdles may be less daunting. The favorable regulatory landscape is expected to enhance competition and innovation within the Orthopedic Regenerative Surgical Product Market, ultimately benefiting patients and healthcare providers alike.
Rising Incidence of Orthopedic Disorders
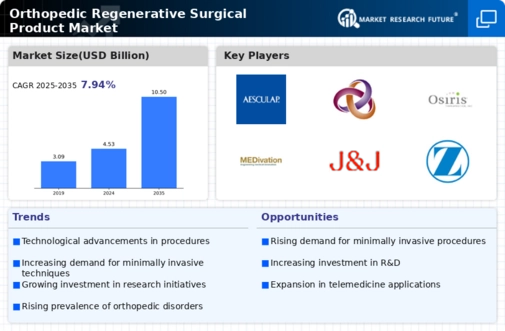
The prevalence of orthopedic disorders, such as osteoarthritis and sports-related injuries, appears to be on the rise, thereby driving the Orthopedic Regenerative Surgical Product Market. According to recent data, approximately 30 million adults in the United States are affected by osteoarthritis alone. This increasing incidence necessitates innovative treatment options, including regenerative surgical products that promote healing and tissue regeneration. As the population ages, the demand for effective orthopedic solutions is likely to escalate, further propelling market growth. The Orthopedic Regenerative Surgical Product Market is thus positioned to benefit from this trend, as healthcare providers seek advanced therapies to address the growing burden of orthopedic conditions.
Growing Demand for Minimally Invasive Procedures
The shift towards minimally invasive surgical techniques is significantly influencing the Orthopedic Regenerative Surgical Product Market. Patients increasingly prefer procedures that offer reduced recovery times and lower risks of complications. This trend is evident in the rising adoption of arthroscopic surgeries and other minimally invasive techniques that utilize regenerative products. Market analysis suggests that the minimally invasive surgery market is expected to grow at a compound annual growth rate of 7.5% over the next five years. As healthcare providers adopt these techniques, the demand for orthopedic regenerative products that facilitate such procedures is likely to increase, thereby driving the market forward.
Increased Investment in Research and Development
Investment in research and development (R&D) within the orthopedic sector is a critical driver for the Orthopedic Regenerative Surgical Product Market. Pharmaceutical companies and medical device manufacturers are allocating substantial resources to develop innovative regenerative solutions. This focus on R&D is expected to yield new products that enhance patient outcomes and expand treatment options. For instance, the orthopedic regenerative market is projected to witness a CAGR of 8% from 2025 to 2030, reflecting the industry's commitment to innovation. As new products emerge from R&D efforts, they are likely to capture market share and stimulate further growth in the Orthopedic Regenerative Surgical Product Market.
Technological Innovations in Regenerative Medicine
Technological advancements in regenerative medicine are transforming the landscape of the Orthopedic Regenerative Surgical Product Market. Innovations such as 3D bioprinting, stem cell therapy, and tissue engineering are enhancing the efficacy of surgical products. For instance, the integration of 3D printing technology allows for the creation of customized implants that match the patient's anatomy, potentially improving surgical outcomes. Furthermore, the market for orthopedic regenerative products is projected to reach USD 10 billion by 2027, indicating a robust growth trajectory fueled by these technological breakthroughs. As these innovations continue to evolve, they are likely to attract investment and research, thereby expanding the Orthopedic Regenerative Surgical Product Market.