Increased Focus on Early Diagnosis
The heightened emphasis on early diagnosis of Non-Radiographic Axial Spondyloarthritis is a crucial driver for the Non-Radiographic Axial Spondyloarthritis Therapeutic Market. Early intervention is associated with better long-term outcomes, prompting healthcare systems to adopt more rigorous screening protocols. The implementation of advanced imaging techniques and biomarkers has facilitated earlier detection, allowing for timely therapeutic interventions. As healthcare providers recognize the importance of addressing this condition in its nascent stages, the demand for effective treatments is expected to surge. This proactive approach not only improves patient quality of life but also contributes to the overall growth of the therapeutic market.
Advancements in Therapeutic Options
Innovations in therapeutic options are significantly influencing the Non-Radiographic Axial Spondyloarthritis Therapeutic Market. The development of targeted biologics and novel small molecules has transformed treatment paradigms, offering patients more effective and personalized care. Recent approvals of medications specifically designed for nr-axSpA have expanded the therapeutic landscape, providing alternatives to traditional non-steroidal anti-inflammatory drugs (NSAIDs). This diversification of treatment options is likely to enhance patient outcomes and adherence, as individuals are more inclined to pursue therapies that align with their specific needs. As research continues to unveil new mechanisms of action, the market is poised for further growth, driven by the introduction of innovative therapies.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies for Non-Radiographic Axial Spondyloarthritis, which serves as a vital driver for the Non-Radiographic Axial Spondyloarthritis Therapeutic Market. Streamlined approval processes and incentives for the development of orphan drugs have encouraged pharmaceutical companies to invest in this area. The expedited review pathways for promising therapies are likely to shorten the time to market, allowing patients to access new treatments more quickly. This regulatory environment not only stimulates competition among manufacturers but also enhances the overall therapeutic landscape, as more options become available to address the diverse needs of patients with nr-axSpA.
Growing Investment in Research and Development
The surge in investment in research and development (R&D) for Non-Radiographic Axial Spondyloarthritis is a significant driver for the Non-Radiographic Axial Spondyloarthritis Therapeutic Market. Pharmaceutical companies are increasingly allocating resources to explore novel therapeutic avenues, including biologics and gene therapies. This trend is indicative of a broader commitment to addressing unmet medical needs within the nr-axSpA population. As R&D efforts yield promising results, the market is likely to witness an influx of new therapies, enhancing treatment options for patients. Furthermore, collaborations between academia and industry are expected to foster innovation, ultimately benefiting the therapeutic landscape.
Rising Prevalence of Non-Radiographic Axial Spondyloarthritis
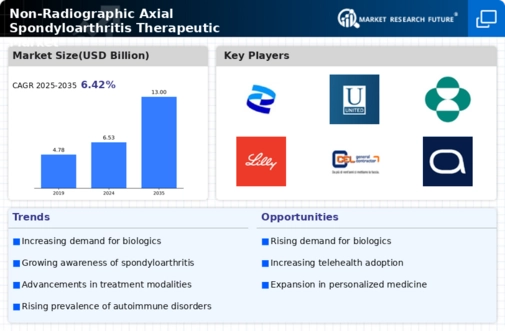
The increasing prevalence of Non-Radiographic Axial Spondyloarthritis (nr-axSpA) is a pivotal driver for the Non-Radiographic Axial Spondyloarthritis Therapeutic Market. Recent estimates suggest that approximately 0.5% to 1% of the population may be affected by this condition, which often goes undiagnosed due to its subtle symptoms. As awareness grows among healthcare professionals and patients alike, the demand for effective therapeutic options is likely to rise. This trend is further supported by the aging population, which is more susceptible to chronic inflammatory diseases. Consequently, the market is expected to expand as more individuals seek treatment, thereby driving innovation and investment in therapeutic solutions tailored for nr-axSpA.