Increased Regulatory Compliance
The Non-PVC IV Bag Market is significantly influenced by stringent regulatory frameworks aimed at ensuring patient safety and product quality. Regulatory bodies are increasingly mandating the use of non-toxic materials in medical devices, including IV bags. This regulatory push is likely to accelerate the adoption of non-PVC IV bags, as manufacturers seek to comply with evolving standards. For instance, the European Union's Medical Device Regulation emphasizes the need for safer alternatives, which could lead to a substantial market shift. As a result, companies that invest in non-PVC technologies may find themselves at a competitive advantage, positioning themselves favorably within the Non-PVC IV Bag Market.
Expansion of Healthcare Infrastructure
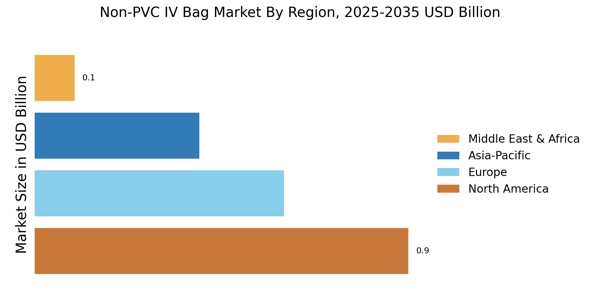
The Non-PVC IV Bag Market is benefiting from the expansion of healthcare infrastructure across various regions. As new hospitals and clinics are established, the demand for medical supplies, including IV bags, is on the rise. This expansion is particularly evident in emerging markets, where investments in healthcare are increasing. The growing number of healthcare facilities is likely to drive the consumption of non-PVC IV bags, as these institutions seek to provide safe and effective treatment options. Additionally, the increasing prevalence of chronic diseases necessitates a reliable supply of IV bags, further propelling the growth of the Non-PVC IV Bag Market. This trend underscores the importance of non-PVC solutions in meeting the evolving needs of healthcare providers.
Rising Demand for Safe Medical Solutions
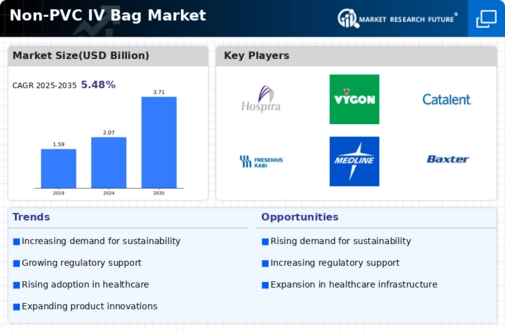
The Non-PVC IV Bag Market is experiencing a notable increase in demand for safer medical solutions. This trend is largely driven by heightened awareness regarding the potential health risks associated with PVC materials, which may leach harmful chemicals into IV fluids. As healthcare providers prioritize patient safety, the shift towards non-PVC alternatives is becoming more pronounced. Market data indicates that the demand for non-PVC IV bags is projected to grow at a compound annual growth rate of approximately 8% over the next five years. This growth reflects a broader commitment to enhancing patient care and minimizing exposure to toxic substances, thereby reinforcing the importance of non-PVC IV bags in modern medical practices.
Technological Innovations in Manufacturing
Technological advancements are playing a pivotal role in the Non-PVC IV Bag Market, facilitating the development of innovative manufacturing processes. These innovations not only enhance the quality and performance of non-PVC IV bags but also reduce production costs. For example, advancements in polymer science have led to the creation of new materials that are both flexible and durable, making them suitable for various medical applications. Furthermore, the integration of automation in manufacturing processes is expected to streamline production, thereby increasing efficiency. As these technologies continue to evolve, they are likely to drive further growth in the Non-PVC IV Bag Market, attracting investments and fostering competition.
Growing Preference for Eco-Friendly Products
The Non-PVC IV Bag Market is witnessing a shift towards eco-friendly products, driven by increasing environmental consciousness among consumers and healthcare providers. Non-PVC IV bags, often made from biodegradable materials, align with sustainability goals and reduce the environmental impact associated with traditional PVC bags. This trend is particularly relevant as healthcare facilities aim to minimize their carbon footprint and adopt greener practices. Market Research Future suggests that the eco-friendly segment of the IV bag market is expected to expand significantly, with non-PVC options leading the way. This growing preference for sustainable solutions is likely to reshape the Non-PVC IV Bag Market, encouraging manufacturers to innovate and adapt.