Growing Geriatric Population
The aging population is a significant driver for the Nitinol Medical Device Market. As individuals age, they are more susceptible to various health issues, including cardiovascular diseases and orthopedic problems, which often require surgical intervention. The World Health Organization estimates that the number of people aged 60 years and older will reach 2 billion by 2050, creating a substantial demand for medical devices that cater to this demographic. Nitinol's biocompatibility and flexibility make it an ideal choice for devices used in elderly patients. Consequently, the Nitinol Medical Device Market is expected to expand as healthcare systems adapt to the needs of an aging population, emphasizing the importance of innovative and effective medical solutions.
Increasing Prevalence of Chronic Diseases
The Nitinol Medical Device Market is experiencing growth due to the rising prevalence of chronic diseases such as cardiovascular disorders, diabetes, and orthopedic conditions. These diseases often necessitate surgical interventions, which in turn drives the demand for advanced medical devices. Nitinol, with its unique properties such as superelasticity and shape memory, is particularly suited for applications in stents, guidewires, and orthopedic implants. According to recent data, the incidence of cardiovascular diseases is projected to rise, leading to an increased need for innovative solutions in the medical device sector. This trend suggests that the Nitinol Medical Device Market will continue to expand as healthcare providers seek effective treatment options for chronic conditions.
Enhanced Focus on Patient-Centric Solutions
The Nitinol Medical Device Market is witnessing a shift towards patient-centric solutions, driven by the increasing emphasis on personalized medicine. Healthcare providers are increasingly recognizing the importance of tailoring treatments to individual patient needs, which has led to a demand for customizable medical devices. Nitinol's unique properties allow for the development of devices that can be adapted to specific anatomical requirements, enhancing their effectiveness. This trend is supported by a growing body of research advocating for personalized approaches in medical treatment. As a result, the Nitinol Medical Device Market is likely to benefit from this focus on patient-centric solutions, fostering innovation and improving patient outcomes.
Technological Advancements in Medical Devices
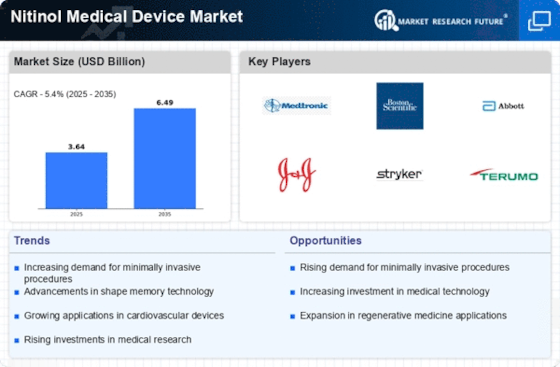
Technological advancements play a pivotal role in the Nitinol Medical Device Market. Innovations in manufacturing processes and material science have enhanced the performance and reliability of Nitinol-based devices. For instance, improvements in 3D printing and laser cutting technologies have enabled the production of complex geometries that were previously unattainable. This has led to the development of more effective and patient-specific devices, thereby increasing their adoption in clinical settings. The market for Nitinol medical devices is projected to grow at a compound annual growth rate (CAGR) of approximately 10% over the next few years, driven by these technological advancements. As a result, the Nitinol Medical Device Market is likely to witness a surge in new product launches and innovations.
Rising Investment in Healthcare Infrastructure
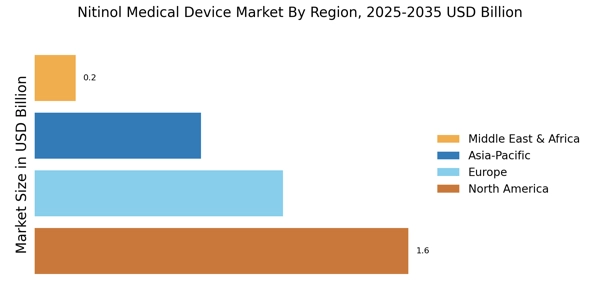
Investment in healthcare infrastructure is a crucial factor influencing the Nitinol Medical Device Market. Governments and private entities are increasingly allocating resources to enhance healthcare facilities, particularly in emerging economies. This investment often includes the procurement of advanced medical technologies, including Nitinol-based devices. As healthcare systems modernize, the demand for minimally invasive surgical options is likely to increase, further propelling the Nitinol Medical Device Market. Reports indicate that healthcare spending is expected to rise significantly, with a focus on improving patient outcomes and reducing recovery times. This trend suggests a favorable environment for the growth of Nitinol medical devices, as they align with the goals of contemporary healthcare systems.