Growing Awareness and Education
The Neurointerventional Device Market is also being driven by growing awareness and education regarding neurological disorders and their treatment options. Healthcare professionals are increasingly educated about the benefits of neurointerventional procedures, leading to higher referral rates for patients. Public awareness campaigns are also playing a role in informing patients about available treatment options, which may lead to earlier diagnosis and intervention. As awareness increases, the demand for neurointerventional devices is expected to rise, as patients seek out advanced treatment options for their neurological conditions.
Increasing Healthcare Expenditure
Rising healthcare expenditure is a significant driver for the Neurointerventional Device Market. As countries allocate more resources to healthcare, there is a corresponding increase in funding for advanced medical technologies. This trend is particularly evident in regions where governments are prioritizing the treatment of neurological disorders. Increased investment in healthcare infrastructure and technology is likely to lead to greater adoption of neurointerventional devices. Furthermore, as healthcare systems evolve, there is a growing emphasis on cost-effective solutions that improve patient outcomes, which neurointerventional devices are well-positioned to provide.
Regulatory Collaborations and Approvals
The Neurointerventional Device Market benefits from ongoing regulatory collaborations that facilitate the approval of new devices. Regulatory bodies are increasingly working with manufacturers to streamline the approval process, ensuring that innovative devices reach the market more efficiently. This collaboration is crucial, as it encourages investment in research and development, leading to the introduction of cutting-edge technologies. The market is witnessing a rise in the number of approved devices, which is likely to enhance competition and drive down costs, ultimately benefiting healthcare providers and patients alike.
Rising Incidence of Neurological Disorders
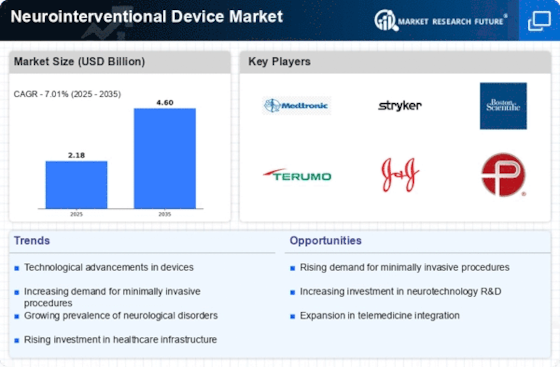
The increasing prevalence of neurological disorders is a primary driver for the Neurointerventional Device Market. Conditions such as stroke, aneurysms, and arteriovenous malformations are becoming more common, necessitating effective treatment options. According to recent data, stroke incidence rates are projected to rise, leading to a greater need for neurointerventional procedures. This trend is further exacerbated by an aging population, which is more susceptible to neurological conditions. As healthcare systems seek to address these challenges, the demand for innovative neurointerventional devices is expected to surge, thereby propelling market growth.
Technological Advancements in Neurointerventional Devices
The Neurointerventional Device Market is experiencing rapid technological advancements that enhance the efficacy and safety of procedures. Innovations such as advanced imaging techniques, robotic-assisted systems, and minimally invasive tools are transforming treatment protocols. For instance, the integration of artificial intelligence in imaging analysis is improving diagnostic accuracy, which is crucial for timely interventions. The market is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 8% in the coming years. These advancements not only improve patient outcomes but also expand the range of treatable conditions, thereby driving demand for neurointerventional devices.