Growing Awareness and Education Initiatives
The increasing awareness and education initiatives surrounding multiple sclerosis are driving the Multiple Sclerosis Treatment Devices Market. Campaigns aimed at educating both healthcare professionals and the general public about MS are gaining momentum. These initiatives are crucial in promoting early diagnosis and treatment, which can significantly improve patient outcomes. As awareness grows, patients are more likely to seek out advanced treatment options, including innovative devices designed for MS management. Furthermore, educational programs are fostering a better understanding of the disease, which may lead to increased advocacy for research and development in treatment technologies. This heightened awareness is expected to contribute positively to market growth as stakeholders respond to the evolving needs of patients.
Increasing Prevalence of Multiple Sclerosis
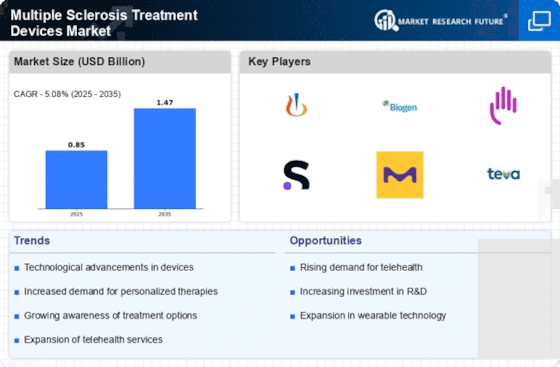
The rising incidence of multiple sclerosis (MS) is a pivotal driver for the Multiple Sclerosis Treatment Devices Market. Recent estimates suggest that approximately 2.3 million individuals are affected by MS worldwide, with a notable increase in diagnoses over the past decade. This growing patient population necessitates the development and availability of effective treatment devices. As awareness of MS expands, healthcare providers are more likely to invest in innovative treatment solutions, thereby propelling market growth. Furthermore, the increasing demand for personalized treatment options is likely to stimulate advancements in device technology, enhancing patient outcomes. The correlation between the prevalence of MS and the demand for treatment devices indicates a robust market potential, as stakeholders seek to address the needs of this expanding demographic.
Regulatory Support for Innovative Treatments
Regulatory support for innovative treatment devices is a key driver in the Multiple Sclerosis Treatment Devices Market. Regulatory bodies are increasingly recognizing the need for expedited approval processes for new therapies and devices aimed at treating MS. This support is crucial in facilitating the introduction of cutting-edge technologies that can significantly enhance patient care. For instance, the implementation of fast-track designations for promising treatment devices can accelerate their availability in the market. As regulatory frameworks evolve to support innovation, manufacturers are likely to invest more in research and development, leading to a broader range of treatment options for patients. This regulatory environment is expected to foster a dynamic market landscape, encouraging the growth of the MS treatment devices sector.
Rising Investment in Healthcare Infrastructure
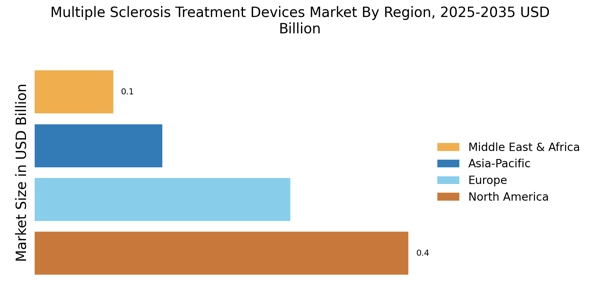
The increasing investment in healthcare infrastructure is a significant driver for the Multiple Sclerosis Treatment Devices Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities and improve access to treatment options for chronic conditions like MS. This trend is particularly evident in regions where healthcare systems are undergoing modernization. Enhanced infrastructure facilitates the distribution and accessibility of advanced treatment devices, thereby meeting the growing demand from patients and healthcare providers. Additionally, the establishment of specialized MS treatment centers is likely to foster innovation and collaboration among stakeholders, further propelling market growth. The commitment to improving healthcare infrastructure suggests a promising outlook for the MS treatment devices market.
Technological Innovations in Treatment Devices
Technological advancements play a crucial role in shaping the Multiple Sclerosis Treatment Devices Market. Innovations such as wearable devices, telehealth solutions, and advanced drug delivery systems are transforming the landscape of MS treatment. For instance, the integration of artificial intelligence and machine learning in treatment devices is enhancing the precision of therapies, allowing for tailored approaches to individual patient needs. The market for these innovative devices is projected to grow significantly, with estimates indicating a compound annual growth rate (CAGR) of over 8% in the coming years. As technology continues to evolve, the potential for improved patient engagement and adherence to treatment regimens becomes increasingly apparent, further driving market expansion.