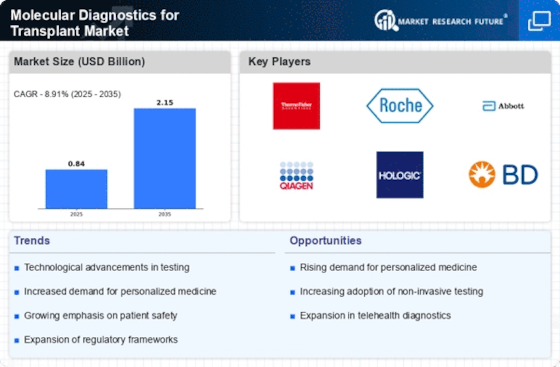
Top Industry Leaders in the Molecular Diagnostics for Transplant Market

Latest Molecular Diagnostics for Transplant Companies Update:
Natera Expanded its Prospera product line with Prospera with Quantification, the first commercially available cfDNA (cell-free DNA) test for non-invasive monitoring of kidney transplant rejection.
CareDx Received FDA approval for AlloMap Heart Care™, a next-generation gene expression test that improves the prediction of cardiac allograft vasculopathy (CAV) development after heart transplant.
Thermo Fisher Scientific Launched the TruSight Transplant One™ Kit, a comprehensive NGS (next-generation sequencing) assay enabling simultaneous analysis of HLA (human leukocyte antigen) typing, CMV (cytomegalovirus) and EBV (Epstein-Barr virus) detection, and chimerism testing in transplant patients.
List of Molecular Diagnostics for Transplant Key companies in the market
- Thermo Fisher Scientific Inc. (US)
- BioMérieux SA (France)
- Cepheid (US)
- Altona Diagnostics GmbH (Germany)
- ELITechGroup (France)
- Hoffmann-La Roche Ltd. (Switzerland)
- Hologic Inc. (US)
- QIAGEN N.V. (Germany)
- Becton, Dickinson, and Company (US)
- Abbott Laboratories, Inc. (US)