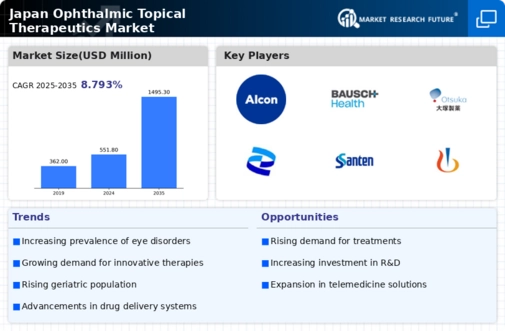
Rising Geriatric Population
The increasing geriatric population in Japan is a pivotal driver for the japan ophthalmic topical therapeutics market. As individuals age, they are more susceptible to various eye disorders, including glaucoma, cataracts, and age-related macular degeneration. According to the Ministry of Health, Labour and Welfare, the proportion of individuals aged 65 and older is projected to reach 30% by 2036. This demographic shift necessitates a greater demand for effective ophthalmic treatments, thereby propelling the growth of the market. Furthermore, the aging population is likely to lead to a higher prevalence of chronic eye conditions, which may require long-term therapeutic interventions. Consequently, pharmaceutical companies are increasingly focusing on developing innovative topical therapies tailored to meet the needs of this demographic, thereby enhancing the overall landscape of the japan ophthalmic topical therapeutics market.
Growing Awareness of Eye Health
The growing awareness of eye health among the Japanese population is a significant driver for the japan ophthalmic topical therapeutics market. Public health campaigns and educational initiatives have been instrumental in informing individuals about the importance of regular eye examinations and early detection of eye disorders. As awareness increases, more individuals are likely to seek preventive care and treatment for existing conditions. This trend is further supported by the rising prevalence of digital eye strain due to increased screen time, prompting individuals to prioritize eye health. Consequently, the demand for effective ophthalmic topical therapeutics is expected to rise as patients become more proactive in managing their eye health. This heightened awareness not only drives market growth but also encourages pharmaceutical companies to develop targeted therapies that address the specific needs of the population, thereby shaping the future of the japan ophthalmic topical therapeutics market.
Increased Healthcare Expenditure
Japan's healthcare expenditure has been on the rise, which serves as a crucial driver for the japan ophthalmic topical therapeutics market. The government has been investing significantly in healthcare infrastructure and services, with total healthcare spending reaching approximately 11% of GDP in recent years. This increase in expenditure is likely to enhance access to ophthalmic care and promote the adoption of advanced therapeutic options. Additionally, the Japanese health insurance system covers a wide range of ophthalmic treatments, making them more accessible to patients. As a result, patients are more inclined to seek timely treatment for eye disorders, thereby boosting the demand for topical therapeutics. This trend indicates a positive outlook for the market, as increased healthcare spending is expected to facilitate the introduction of innovative therapies and improve patient outcomes in the japan ophthalmic topical therapeutics market.
Technological Innovations in Ophthalmology
Technological advancements in ophthalmology are significantly influencing the japan ophthalmic topical therapeutics market. Innovations such as sustained-release drug delivery systems and nanotechnology are enhancing the efficacy and safety of topical treatments. For instance, the introduction of drug-eluting contact lenses and biodegradable implants has the potential to improve patient compliance and therapeutic outcomes. The Japanese government has been actively promoting research and development in this field, with initiatives aimed at fostering collaboration between academia and industry. As a result, the market is witnessing a surge in the introduction of novel therapeutic agents that leverage these technologies. This trend not only addresses the growing demand for effective treatments but also positions Japan as a leader in ophthalmic innovation, thereby driving the growth of the japan ophthalmic topical therapeutics market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a key driver for the japan ophthalmic topical therapeutics market. The Pharmaceuticals and Medical Devices Agency (PMDA) in Japan has been streamlining the approval process for new ophthalmic drugs, thereby encouraging the development of novel therapeutic options. This supportive regulatory environment is particularly beneficial for companies focusing on research and development of cutting-edge treatments. The expedited review process for breakthrough therapies allows for quicker access to the market, which is crucial in addressing urgent medical needs. Furthermore, the Japanese government has been promoting initiatives to foster innovation in the pharmaceutical sector, which is likely to result in a diverse range of new products entering the market. This regulatory landscape not only enhances the competitiveness of the japan ophthalmic topical therapeutics market but also ensures that patients have access to the latest advancements in eye care.