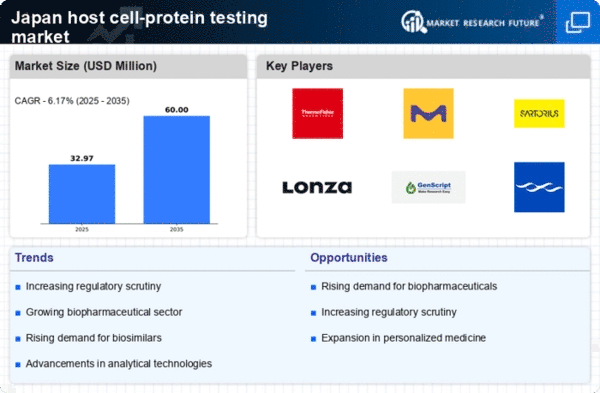
Growing Regulatory Scrutiny
The regulatory landscape in Japan is evolving, with increased scrutiny on biopharmaceutical products driving demand for host cell-protein testing. Regulatory bodies are emphasizing the need for comprehensive testing to ensure that products are free from contaminants that could compromise patient safety. In 2025, it is anticipated that regulatory compliance costs will rise by 12%, prompting companies to prioritize host cell-protein testing as part of their quality assurance strategies. This heightened regulatory focus is likely to stimulate growth in the host cell protein testing market, as manufacturers seek to align with stringent guidelines and maintain their competitive edge.
Expansion of Biotechnology Firms
The proliferation of biotechnology firms in Japan is a significant factor influencing the host cell-protein-testing market. As new companies emerge, particularly in the fields of gene therapy and monoclonal antibodies, the demand for host cell-protein testing is expected to increase. In 2025, the number of biotechnology startups in Japan is projected to grow by 20%, creating a larger market for testing services. These firms often require specialized testing to validate their products, thereby driving the need for advanced host cell-protein testing solutions. This trend indicates a promising future for the host cell-protein-testing market, as it adapts to the evolving needs of a dynamic biotechnology landscape.
Increased Focus on Quality Control
Quality control remains a critical aspect of biopharmaceutical manufacturing in Japan, significantly influencing the host cell protein testing market. As companies strive to enhance product quality, the implementation of stringent testing protocols for host cell proteins is becoming increasingly vital. The Japanese pharmaceutical industry has seen a shift towards more comprehensive quality assurance measures, with an estimated 15% increase in quality control budgets in 2025. This trend underscores the importance of reliable testing methods to identify impurities and ensure compliance with regulatory requirements. As a result, the host cell-protein-testing market is likely to experience growth driven by the heightened emphasis on quality assurance in biopharmaceutical production.
Rising Biopharmaceutical Production
The increasing demand for biopharmaceuticals in Japan is a key driver for the host cell protein testing market. As the biopharmaceutical sector expands, the need for rigorous testing of host cell proteins becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in Japan is projected to reach approximately $20 billion, indicating a robust growth trajectory. This surge necessitates advanced testing methodologies to detect and quantify host cell proteins, which can potentially impact therapeutic outcomes. Consequently, companies are investing in innovative testing solutions to meet regulatory standards and consumer expectations, thereby propelling the host cell-protein-testing market forward.
Advancements in Analytical Technologies
Technological innovations in analytical methods are reshaping the landscape of the host cell protein testing market. In Japan, the adoption of advanced techniques such as mass spectrometry and high-performance liquid chromatography (HPLC) is on the rise. These technologies offer enhanced sensitivity and specificity, enabling more accurate detection of host cell proteins. The market for analytical instruments is expected to grow by approximately 10% annually, reflecting the increasing investment in state-of-the-art testing solutions. This trend suggests that as analytical capabilities improve, the host cell-protein-testing market will benefit from more efficient and reliable testing processes, ultimately leading to better product quality and safety.