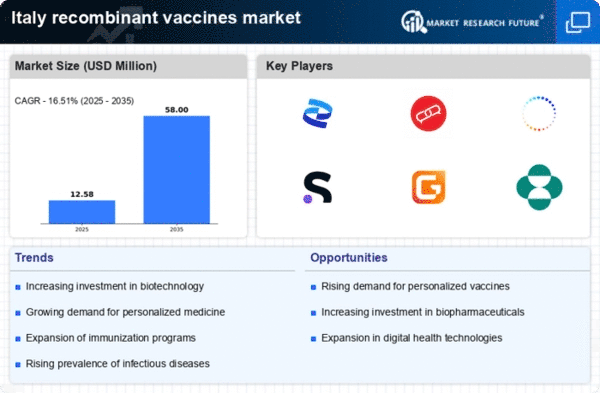
Focus on Personalized Medicine
The trend towards personalized medicine is emerging as a significant driver for the recombinant vaccines market in Italy. As healthcare becomes more tailored to individual patient needs, there is a growing interest in developing vaccines that can be customized based on genetic and environmental factors. This shift is supported by advancements in genomics and biotechnology, which are enabling the creation of more effective and targeted vaccines. In 2025, the Italian government is expected to invest €200 million in initiatives aimed at integrating personalized medicine into public health strategies. This focus on personalized approaches is likely to enhance the efficacy of vaccines, thereby driving growth in the recombinant vaccines market.
Growing Biopharmaceutical Sector
The biopharmaceutical sector in Italy is expanding rapidly, which is positively impacting the recombinant vaccines market. With a market size projected to reach €10 billion by 2026, the biopharmaceutical industry is increasingly focusing on vaccine development. This growth is attributed to advancements in biotechnology and the increasing number of biopharmaceutical companies entering the market. The recombinant vaccines market benefits from this trend as these companies invest in innovative vaccine technologies. Furthermore, the collaboration between biopharmaceutical firms and research institutions is likely to enhance the development pipeline for recombinant vaccines, thereby driving market growth in Italy.
Increased Public-Private Partnerships
Public-private partnerships (PPPs) are becoming more prevalent in Italy, particularly in the healthcare sector, which is influencing the recombinant vaccines market. These collaborations facilitate the sharing of resources, expertise, and funding, leading to more efficient vaccine development processes. In 2025, several PPP initiatives have been launched, focusing on enhancing vaccine accessibility and distribution. This collaborative approach is expected to streamline the regulatory approval process and reduce time-to-market for new recombinant vaccines. As a result, the recombinant vaccines market is likely to benefit from improved vaccine availability and affordability, ultimately enhancing public health outcomes in Italy.
Investment in Research and Development
Investment in research and development (R&D) is a crucial driver for the recombinant vaccines market in Italy. The Italian government, alongside private entities, has been increasing funding for vaccine research, with an estimated €300 million dedicated to innovative vaccine technologies in 2025. This financial support is aimed at fostering advancements in recombinant vaccine formulations and delivery systems. The collaboration between academic institutions and pharmaceutical companies is also enhancing the R&D landscape, leading to the development of novel vaccines that address emerging health threats. As a result, the recombinant vaccines market is likely to experience accelerated growth, driven by the introduction of new and effective vaccine candidates.
Rising Demand for Preventive Healthcare
The increasing focus on preventive healthcare in Italy is driving the recombinant vaccines market. As healthcare systems evolve, there is a notable shift towards vaccination as a primary strategy for disease prevention. This trend is supported by public awareness campaigns that emphasize the importance of immunization. In 2025, the Italian government allocated approximately €500 million to enhance vaccination programs, which is expected to boost the recombinant vaccines market significantly. The growing recognition of the economic benefits of preventing diseases through vaccination is likely to further propel market growth. Additionally, the rising incidence of infectious diseases in Italy underscores the need for effective vaccines, thereby creating a favorable environment for the recombinant vaccines market.