Rising Demand for Rapid Testing
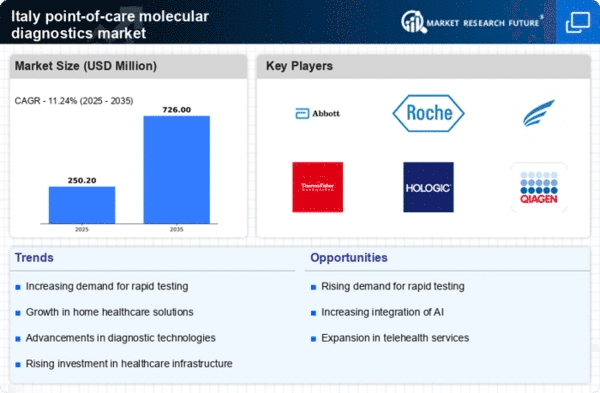
The increasing need for rapid diagnostic solutions is a key driver in the point of-care-molecular-diagnostics market. In Italy, healthcare providers are increasingly prioritizing quick and accurate testing methods to enhance patient outcomes. This demand is reflected in the market's growth, which is projected to reach approximately €1.5 billion by 2026. The ability to deliver results within minutes rather than days is particularly appealing in emergency and outpatient settings. As healthcare systems strive for efficiency, the adoption of point-of-care testing is likely to expand, driven by both patient expectations and clinical requirements. This trend indicates a shift towards decentralized healthcare, where diagnostics are performed closer to the patient, thereby improving access and reducing the burden on centralized laboratories.
Government Initiatives and Funding
Government initiatives and funding aimed at enhancing healthcare infrastructure are pivotal drivers of the point of-care-molecular-diagnostics market. In Italy, the government has been actively investing in healthcare technologies to improve diagnostic capabilities and patient care. Recent funding programs have allocated millions of euros to support the development and implementation of innovative diagnostic solutions. This financial backing is expected to stimulate market growth, as it encourages research and development in molecular diagnostics. Additionally, public health campaigns promoting early detection and treatment of diseases are likely to further boost the demand for point-of-care testing solutions. Such initiatives indicate a commitment to improving healthcare outcomes and accessibility across the country.
Integration of Advanced Technologies
The integration of advanced technologies such as artificial intelligence (AI) and machine learning into the point of-care-molecular-diagnostics market is transforming the landscape of medical diagnostics in Italy. These technologies enhance the accuracy and speed of diagnostic tests, allowing for more precise patient management. For instance, AI algorithms can analyze complex data sets from molecular tests, leading to improved decision-making in clinical settings. The market is expected to witness a compound annual growth rate (CAGR) of around 10% over the next five years, driven by these technological advancements. Furthermore, the incorporation of mobile health applications is facilitating remote monitoring and diagnostics, making healthcare more accessible to the population. This technological evolution is likely to redefine the operational dynamics of the healthcare sector.
Growing Awareness of Infectious Diseases
The increasing awareness of infectious diseases is a significant driver in the point of-care-molecular-diagnostics market. In Italy, public health campaigns and educational initiatives have heightened the population's understanding of the importance of early detection and treatment of infectious diseases. This awareness is leading to a greater demand for rapid and accurate diagnostic tests, which are essential for effective disease management. The market is anticipated to grow by around 12% over the next few years, as healthcare providers respond to this demand by integrating point-of-care testing into their services. The focus on infectious diseases underscores the need for timely interventions, thereby reinforcing the role of molecular diagnostics in public health strategies.
Increased Focus on Personalized Medicine
The growing emphasis on personalized medicine is significantly influencing the point of-care-molecular-diagnostics market. In Italy, healthcare providers are increasingly recognizing the importance of tailoring treatments to individual patient profiles, which necessitates precise diagnostic tools. This trend is expected to drive the market's expansion, as molecular diagnostics play a crucial role in identifying specific biomarkers that guide treatment decisions. The market is projected to grow by approximately 15% annually, reflecting the rising adoption of personalized approaches in healthcare. As patients demand more customized care, the integration of molecular diagnostics into routine clinical practice is likely to become more prevalent, thereby enhancing treatment efficacy and patient satisfaction.