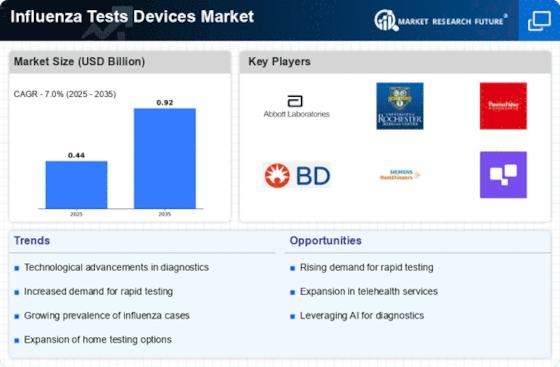
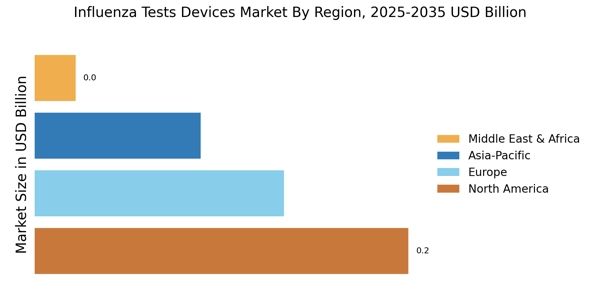
Rising Incidence of Influenza
The increasing incidence of influenza infections is a primary driver for the Influenza Tests Devices Market. Seasonal outbreaks of influenza lead to heightened awareness and demand for effective diagnostic tools. According to health authorities, influenza affects millions annually, resulting in significant morbidity and mortality. This persistent threat necessitates the availability of reliable testing devices to facilitate timely diagnosis and treatment. As healthcare systems strive to manage influenza outbreaks, the demand for advanced testing devices is likely to surge. The Influenza Tests Devices Market is thus positioned to benefit from this trend, as healthcare providers seek to enhance their diagnostic capabilities to combat the rising burden of influenza.
Rising Awareness of Influenza Testing
Rising awareness of the importance of influenza testing among healthcare professionals and the general public is a significant driver for the Influenza Tests Devices Market. Educational campaigns and information dissemination have led to a better understanding of the benefits of timely diagnosis and treatment of influenza. This heightened awareness encourages individuals to seek testing when experiencing symptoms, thereby increasing demand for testing devices. Furthermore, healthcare providers are increasingly recognizing the value of accurate influenza diagnostics in managing patient care. As awareness continues to grow, the Influenza Tests Devices Market is likely to expand, driven by the need for effective testing solutions that align with public health objectives.
Growing Focus on Preventive Healthcare
The growing emphasis on preventive healthcare is significantly influencing the Influenza Tests Devices Market. As populations become more health-conscious, there is an increasing demand for early detection and prevention strategies. Influenza testing plays a crucial role in identifying infections before they escalate, thereby reducing transmission rates. This shift towards preventive measures is supported by public health campaigns and initiatives aimed at increasing vaccination rates and promoting awareness of influenza symptoms. Consequently, the Influenza Tests Devices Market is likely to experience growth as healthcare providers invest in testing devices that facilitate early diagnosis and intervention, ultimately improving patient outcomes.
Increased Government Initiatives and Funding
Increased government initiatives and funding for influenza surveillance and control programs are pivotal drivers for the Influenza Tests Devices Market. Governments worldwide are recognizing the importance of robust influenza monitoring systems to mitigate the impact of outbreaks. Funding for research and development of testing devices is on the rise, as authorities aim to enhance public health responses. This financial support facilitates the introduction of innovative testing technologies and expands access to diagnostic tools in underserved areas. Consequently, the Influenza Tests Devices Market stands to benefit from these initiatives, as enhanced funding translates into improved testing capabilities and greater availability of devices in the market.
Technological Innovations in Testing Devices
Technological innovations are transforming the Influenza Tests Devices Market, leading to the development of more efficient and accurate testing solutions. Advances in molecular diagnostics, such as polymerase chain reaction (PCR) and rapid antigen tests, have enhanced the speed and reliability of influenza detection. These innovations not only improve diagnostic accuracy but also reduce the time required for results, which is critical during peak influenza seasons. The integration of digital technologies, such as telemedicine and mobile health applications, further supports the accessibility of testing devices. As a result, the Influenza Tests Devices Market is poised for growth, driven by the demand for cutting-edge diagnostic tools that meet the evolving needs of healthcare providers.