Cost-Effectiveness of Outsourcing
Outsourcing manufacturing processes has become a strategic approach for many companies in the ivd contract-manufacturing market. By leveraging the expertise of specialized manufacturers, companies can reduce operational costs and enhance efficiency. In India, the cost of labor is relatively lower compared to developed nations, making it an attractive destination for contract manufacturing. This cost advantage allows companies to allocate resources more effectively, potentially increasing their market share. As a result, the ivd contract-manufacturing market is witnessing a shift towards outsourcing, which is expected to continue driving growth in the coming years.
Innovation in Product Development
The ivd contract-manufacturing market is experiencing a surge in innovation, driven by advancements in technology and research. Companies are increasingly focusing on developing novel diagnostic products that meet the evolving needs of healthcare providers and patients. This innovation is not only enhancing the accuracy and speed of diagnostics but also expanding the range of tests available. For instance, the introduction of point-of-care testing devices is revolutionizing the way diagnostics are conducted. As a result, the ivd contract-manufacturing market is likely to see a rise in demand for manufacturers who can support the development of these cutting-edge products.
Government Initiatives and Support
The Indian government has been actively promoting the healthcare sector, including the ivd contract-manufacturing market, through various initiatives and policies. Programs aimed at enhancing healthcare infrastructure and increasing access to diagnostic services are likely to create a favorable environment for market growth. Additionally, the government is encouraging foreign investment in the healthcare sector, which could lead to increased collaboration between local manufacturers and international companies. This supportive regulatory framework is expected to bolster the ivd contract-manufacturing market, making it an attractive option for both domestic and foreign players.
Rising Demand for Diagnostic Testing
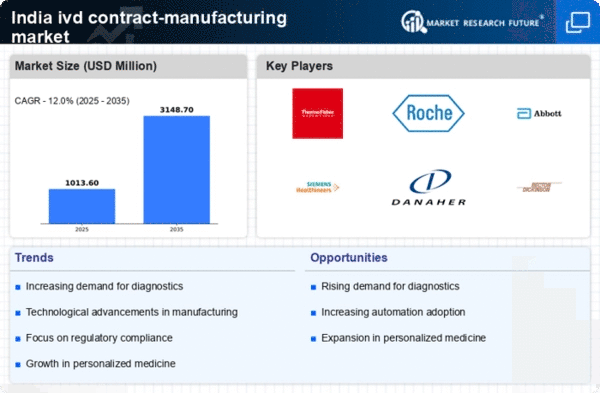
The increasing prevalence of chronic diseases and the growing awareness of preventive healthcare are driving the demand for diagnostic testing in India. This trend is particularly evident in the ivd contract-manufacturing market, where the need for accurate and timely diagnostics is paramount. According to recent estimates, the Indian diagnostic market is projected to reach approximately $10 billion by 2025, with a significant portion attributed to in vitro diagnostics. As healthcare providers seek reliable partners for manufacturing, the ivd contract-manufacturing market is likely to experience substantial growth, fueled by the need for innovative diagnostic solutions that cater to diverse healthcare needs.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is reshaping the landscape of the ivd contract-manufacturing market in India. As healthcare providers increasingly recognize the importance of tailored treatments, the demand for diagnostic tests that can guide personalized therapy is on the rise. This trend is prompting manufacturers to develop more sophisticated testing solutions that can provide insights into individual patient profiles. Consequently, the ivd contract-manufacturing market is likely to expand as companies seek to innovate and produce tests that align with the principles of personalized medicine, thereby enhancing patient outcomes.