Advancements in Biotechnology
Technological advancements in biotechnology are significantly influencing the gene therapy market. Innovations in gene editing techniques, such as CRISPR-Cas9, are enabling more precise and effective therapies. These advancements are not only enhancing the efficacy of gene therapies but also reducing associated costs, making them more accessible to the Indian population. The biotechnology sector in India is experiencing rapid growth, with investments reaching approximately $11 billion in 2025. This influx of capital is likely to foster the development of new gene therapies, thereby expanding the market. Additionally, collaborations between academic institutions and biotech firms are expected to accelerate research and development efforts, further propelling the gene therapy market forward.
Growing Awareness and Acceptance
The rising awareness and acceptance of gene therapy among healthcare professionals and patients are driving the market's growth. As educational campaigns and outreach programs increase, more individuals are becoming informed about the potential benefits of gene therapy. This shift in perception is crucial, as it encourages patients to seek out these innovative treatments. Additionally, healthcare providers are becoming more knowledgeable about gene therapy options, leading to increased referrals and prescriptions. The gene therapy market is likely to benefit from this growing acceptance, as more patients are willing to explore advanced treatment options for their conditions. This trend may also lead to a more robust pipeline of gene therapies being developed and brought to market.
Government Initiatives and Funding
Government initiatives aimed at promoting healthcare innovation are playing a crucial role in the gene therapy market. The Indian government has launched various programs to support research and development in biotechnology, with funding allocations increasing by approximately 15% in recent years. These initiatives are designed to encourage the development of advanced therapies, including gene therapy. By providing financial support and creating a favorable regulatory environment, the government is likely to stimulate growth in the gene therapy market. Furthermore, public-private partnerships are emerging, which could enhance the capabilities of local firms to develop and commercialize gene therapies, ultimately benefiting patients in need.
Rising Prevalence of Genetic Disorders
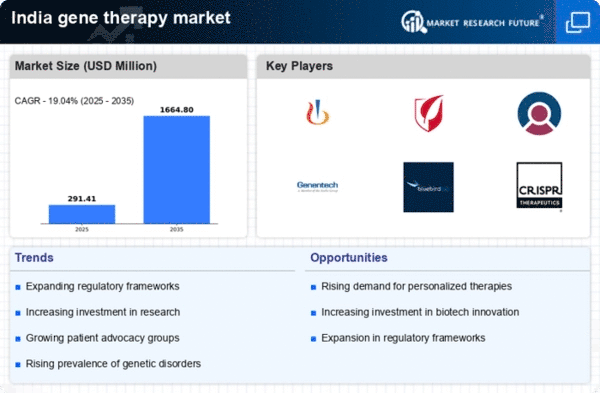
The increasing incidence of genetic disorders in India is a primary driver for the gene therapy market. With an estimated 6-8 million people affected by genetic conditions, the demand for innovative treatment options is surging. This growing patient population necessitates the development of targeted therapies, which gene therapy can provide. The Indian healthcare system is gradually recognizing the potential of gene therapy as a viable treatment option, leading to increased investments in research and clinical trials. As awareness of genetic disorders rises, healthcare providers are more likely to explore gene therapy solutions, thereby expanding the market. Furthermore, the government is likely to support initiatives aimed at addressing genetic disorders, which could further stimulate growth in the gene therapy market.
Increased Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, research institutions, and healthcare providers, is emerging as a key driver for the gene therapy market. These partnerships facilitate the sharing of knowledge, resources, and expertise, which can accelerate the development of innovative therapies. In India, collaborative efforts are becoming more common, with several joint ventures and alliances being formed to advance gene therapy research. Such collaborations can lead to more efficient clinical trials and faster regulatory approvals, ultimately benefiting patients. The gene therapy market is likely to see enhanced growth as these partnerships continue to evolve, fostering an environment conducive to innovation and development.