Government Initiatives and Funding
Government initiatives aimed at improving women's health are significantly impacting the Global Hpv Testing Pap Test Market Industry. Many countries are implementing national screening programs and providing funding for HPV vaccination and testing. These initiatives are designed to reduce the incidence of cervical cancer and promote preventive healthcare. For instance, increased funding for public health campaigns and subsidized testing programs can lead to higher participation rates in screenings. This supportive environment is likely to contribute to a compound annual growth rate of 12.03% from 2025 to 2035, underscoring the importance of governmental support in market expansion.
Rising Incidence of Cervical Cancer
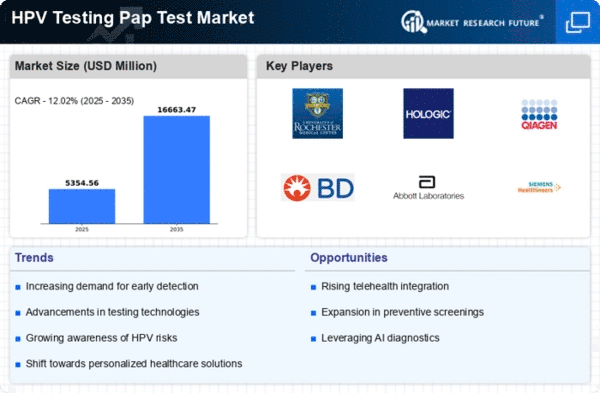
The increasing incidence of cervical cancer globally is a significant driver for the Global Hpv Testing Pap Test Market Industry. As more women are diagnosed with cervical cancer, the demand for effective screening methods, including HPV testing and Pap tests, rises. This trend is particularly evident in regions with limited access to healthcare, where awareness and screening rates are lower. The urgency to address this health crisis is prompting healthcare systems to prioritize HPV testing, thereby fueling market growth. The projected market value of 4.78 USD Billion in 2024 reflects this pressing need for effective cancer prevention strategies.
Technological Advancements in Testing Methods
Innovations in testing methodologies are driving the Global Hpv Testing Pap Test Market Industry forward. The introduction of more accurate and efficient testing techniques, such as liquid-based cytology and HPV DNA testing, enhances the reliability of results. These advancements not only improve patient outcomes but also streamline the testing process, making it more accessible. As healthcare providers adopt these technologies, the market is expected to witness substantial growth, with projections indicating an increase to 16.7 USD Billion by 2035, highlighting the potential of technological integration in healthcare.
Increasing Awareness of HPV and Cervical Cancer
The Global Hpv Testing Pap Test Market Industry is experiencing growth due to heightened awareness regarding HPV and its association with cervical cancer. Educational campaigns and initiatives by health organizations are effectively informing the public about the importance of regular screenings. This awareness is crucial, as it encourages women to undergo HPV testing and Pap tests, leading to early detection and treatment. As a result, the market is projected to reach 4.78 USD Billion in 2024, reflecting a growing recognition of the need for preventive healthcare measures.
Integration of HPV Testing into Routine Healthcare
The integration of HPV testing into routine healthcare practices is transforming the Global Hpv Testing Pap Test Market Industry. Healthcare providers are increasingly recommending HPV testing alongside traditional Pap tests as part of regular gynecological examinations. This shift is driven by the recognition of HPV's role in cervical cancer development and the need for comprehensive screening strategies. As more healthcare systems adopt this integrated approach, the market is expected to grow significantly, potentially reaching 16.7 USD Billion by 2035. This trend indicates a broader acceptance of HPV testing as a standard component of women's health.