Rising Demand for Biopharmaceuticals
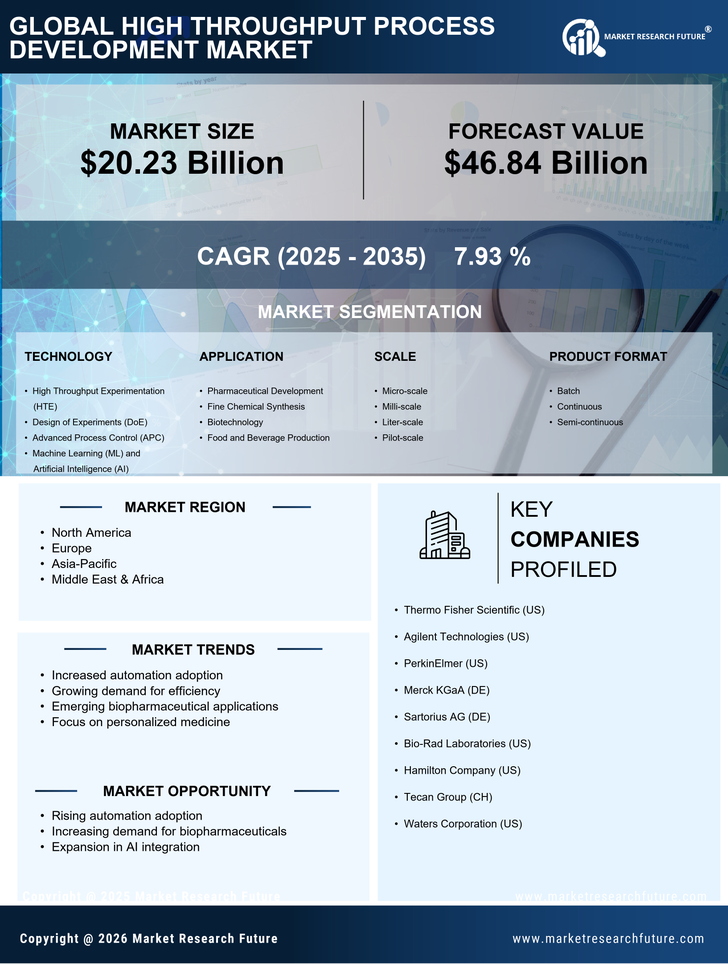
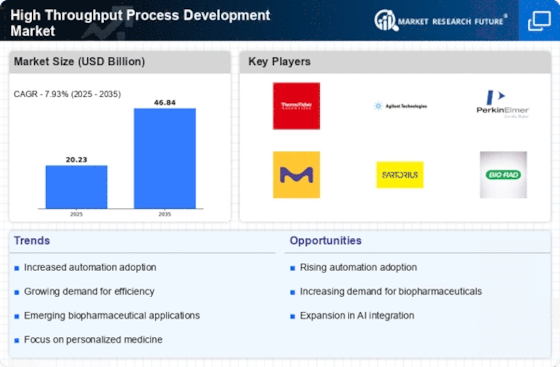
The increasing demand for biopharmaceuticals is a primary driver of the High Throughput Process Development Market. As the biopharmaceutical sector continues to expand, driven by advancements in biotechnology and personalized medicine, the need for efficient and rapid process development becomes critical. According to recent data, the biopharmaceutical market is projected to reach approximately 500 billion USD by 2025. This surge necessitates high throughput methodologies to streamline the development of new therapeutics, thereby enhancing productivity and reducing time-to-market. Companies are increasingly investing in high throughput technologies to meet regulatory requirements and to ensure the scalability of their processes. Consequently, the High Throughput Process Development Market is likely to experience substantial growth as organizations seek to optimize their production capabilities in response to this rising demand.
Growing Focus on Personalized Medicine
The growing focus on personalized medicine is reshaping the High Throughput Process Development Market. As healthcare moves towards more tailored treatment options, the demand for high throughput methodologies that can efficiently develop and test personalized therapies is increasing. This shift is supported by the need for rapid screening of patient-specific responses to various treatments. The market for personalized medicine is projected to reach over 2 trillion USD by 2025, highlighting the urgency for innovative process development solutions. High throughput techniques enable researchers to analyze vast datasets and identify optimal treatment pathways for individual patients. Consequently, the High Throughput Process Development Market is likely to see a surge in investment and innovation as stakeholders strive to meet the needs of this evolving healthcare landscape.
Technological Advancements in Automation
Technological advancements in automation are significantly influencing the High Throughput Process Development Market. Automation technologies, including robotics and artificial intelligence, are being integrated into laboratory processes to enhance efficiency and accuracy. These innovations allow for the simultaneous processing of multiple experiments, thereby accelerating the development timeline. The market for laboratory automation is expected to grow at a compound annual growth rate of over 10% in the coming years. This trend indicates a shift towards more automated solutions, which not only reduce human error but also enable researchers to focus on data analysis and interpretation. As automation becomes more prevalent, the High Throughput Process Development Market is poised to benefit from increased adoption of these technologies, leading to improved throughput and reduced operational costs.
Increased Investment in Research and Development
Increased investment in research and development (R&D) is a significant driver of the High Throughput Process Development Market. Organizations across various sectors, particularly in pharmaceuticals and biotechnology, are allocating substantial resources to R&D to foster innovation and maintain competitive advantage. In 2025, R&D spending in the pharmaceutical industry is expected to exceed 200 billion USD, reflecting a commitment to developing new therapies and improving existing ones. High throughput process development plays a crucial role in this context, as it allows for the rapid evaluation of multiple compounds and formulations. This efficiency not only accelerates the discovery process but also enhances the likelihood of successful outcomes. As R&D investments continue to rise, the High Throughput Process Development Market is likely to expand in tandem, driven by the need for faster and more effective development processes.
Regulatory Pressures and Compliance Requirements
Regulatory pressures and compliance requirements are increasingly shaping the High Throughput Process Development Market. As regulatory bodies impose stricter guidelines on the development and approval of new drugs, companies are compelled to adopt high throughput methodologies that ensure compliance while maintaining efficiency. The need for robust data generation and validation processes is paramount, as regulatory agencies demand comprehensive evidence of safety and efficacy. This trend is expected to drive the adoption of high throughput technologies, which facilitate the generation of large datasets necessary for regulatory submissions. Furthermore, the market is likely to see a rise in demand for platforms that streamline compliance processes, thereby reducing the burden on organizations. As a result, the High Throughput Process Development Market is positioned to grow in response to these evolving regulatory landscapes.