Rising Incidence of Hepatitis E
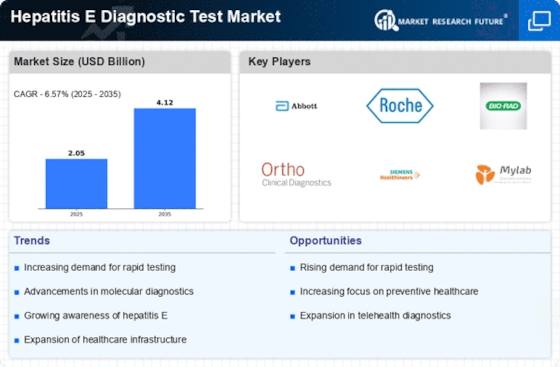
The increasing incidence of Hepatitis E infections is a primary driver for the Hepatitis E Diagnostic Test Market. Reports indicate that Hepatitis E is becoming more prevalent, particularly in regions with inadequate sanitation and water quality. The World Health Organization has noted that Hepatitis E is endemic in many developing countries, leading to a heightened demand for effective diagnostic tests. As the number of reported cases rises, healthcare providers are compelled to seek reliable diagnostic solutions to manage and control outbreaks. This trend suggests that the market for Hepatitis E diagnostic tests is likely to expand, as healthcare systems prioritize the identification and treatment of this viral infection. Consequently, the rising incidence of Hepatitis E is expected to significantly influence the growth trajectory of the Hepatitis E Diagnostic Test Market.
Government Initiatives and Funding
Government initiatives aimed at combating infectious diseases are significantly impacting the Hepatitis E Diagnostic Test Market. Many countries are implementing national health programs that focus on improving diagnostic capabilities for Hepatitis E. These initiatives often include funding for research and development of new diagnostic tests, as well as public health campaigns to raise awareness about the disease. For instance, increased budget allocations for infectious disease control have been observed in various nations, which directly supports the development and distribution of Hepatitis E diagnostic tests. Such government backing not only enhances the availability of testing but also encourages collaboration between public health entities and private sector companies. This synergy is likely to foster innovation and expand the Hepatitis E Diagnostic Test Market.
Technological Innovations in Testing
Technological advancements in diagnostic testing are propelling the Hepatitis E Diagnostic Test Market forward. Innovations such as rapid testing kits, molecular diagnostics, and point-of-care testing are enhancing the accuracy and speed of Hepatitis E detection. These advancements not only improve patient outcomes but also facilitate timely public health responses to outbreaks. The introduction of more sophisticated testing methods, including serological and nucleic acid tests, has expanded the diagnostic capabilities available to healthcare providers. As a result, the market is witnessing an influx of new products that cater to diverse healthcare settings. The integration of technology in diagnostics is likely to attract investment and research, further stimulating growth in the Hepatitis E Diagnostic Test Market.
Increased Global Travel and Migration
Increased The Hepatitis E Diagnostic Test Industry. As people move across borders, the risk of spreading infectious diseases, including Hepatitis E, escalates. Travelers and migrants may encounter regions where Hepatitis E is endemic, leading to a higher likelihood of infection. This dynamic creates a pressing need for effective diagnostic tests to identify and manage cases promptly. Healthcare providers are increasingly recognizing the importance of screening travelers for Hepatitis E, particularly those returning from high-risk areas. The rise in international travel and migration patterns is likely to drive demand for Hepatitis E diagnostic tests, as public health authorities aim to mitigate the risks associated with cross-border disease transmission. Thus, the impact of increased The Hepatitis E Diagnostic Test Industry is expected to be substantial.
Growing Demand for Preventive Healthcare
The growing emphasis on preventive healthcare is driving the Hepatitis E Diagnostic Test Market. As healthcare systems worldwide shift towards preventive measures, the importance of early detection and diagnosis of infectious diseases like Hepatitis E is becoming increasingly recognized. This trend is reflected in the rising demand for diagnostic tests that can identify infections before they lead to severe health complications. Preventive healthcare initiatives often include routine screening programs, which are likely to incorporate Hepatitis E testing, especially in high-risk populations. The proactive approach to health management is expected to create a robust market for Hepatitis E diagnostic tests, as healthcare providers seek to implement comprehensive screening strategies. Consequently, the focus on preventive healthcare is anticipated to significantly contribute to the growth of the Hepatitis E Diagnostic Test Market.