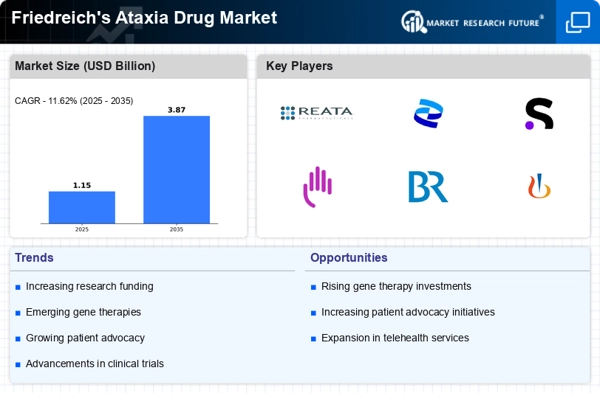
Advancements in Genetic Research
Advancements in genetic research are significantly influencing the Friedreich's Ataxia Drug Market. The identification of the FXN gene, responsible for Friedreich's Ataxia, has opened new avenues for targeted therapies. Researchers are exploring gene editing techniques and other innovative approaches to correct the underlying genetic defects. This progress not only enhances the understanding of the disease but also fosters the development of novel treatment modalities. As a result, the Friedreich's Ataxia Drug Market is witnessing an influx of clinical trials aimed at evaluating the efficacy of these emerging therapies. The potential for breakthroughs in genetic interventions may reshape treatment paradigms and improve patient outcomes.
Growing Patient Advocacy and Awareness
Growing patient advocacy and awareness initiatives are playing a pivotal role in shaping the Friedreich's Ataxia Drug Market. Advocacy groups are actively working to raise awareness about the disease, its symptoms, and the need for effective treatments. These efforts are not only educating the public but also influencing policymakers to prioritize research funding and support for Friedreich's Ataxia. As awareness increases, more patients are likely to seek medical attention, leading to higher diagnosis rates. This, in turn, could stimulate demand for therapies within the Friedreich's Ataxia Drug Market, as stakeholders respond to the needs of an informed patient population.
Rising Prevalence of Friedreich's Ataxia
The increasing prevalence of Friedreich's Ataxia is a notable driver for the Friedreich's Ataxia Drug Market. Recent estimates suggest that the condition affects approximately 1 in 50,000 individuals, leading to a growing patient population in need of effective treatments. This rising incidence is prompting pharmaceutical companies to invest in research and development, aiming to address the unmet medical needs of patients. As awareness of the disease expands, more individuals are being diagnosed, which could potentially lead to an increase in demand for therapeutic options. Consequently, the Friedreich's Ataxia Drug Market is likely to experience growth as stakeholders recognize the necessity for innovative solutions to manage this debilitating condition.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a key driver in the Friedreich's Ataxia Drug Market. Regulatory agencies are increasingly adopting frameworks that facilitate the expedited approval of treatments for rare diseases. This supportive environment encourages pharmaceutical companies to invest in the development of novel therapies for Friedreich's Ataxia. The potential for faster market access and reduced development timelines may incentivize more players to enter the Friedreich's Ataxia Drug Market. As a result, patients may benefit from a broader array of treatment options, enhancing the overall landscape of care for this challenging condition.
Increased Investment in Rare Disease Research
The Friedreich's Ataxia Drug Market is benefiting from increased investment in rare disease research. Governments and private organizations are recognizing the importance of addressing rare conditions, leading to enhanced funding opportunities for research initiatives. This financial support is crucial for the development of new therapies and clinical trials focused on Friedreich's Ataxia. In recent years, funding for rare disease research has surged, with billions allocated to support innovative projects. This trend is likely to accelerate the pace of discovery and development within the Friedreich's Ataxia Drug Market, ultimately resulting in more treatment options for patients.