Supportive Regulatory Framework
The regulatory environment in France is becoming increasingly supportive of the recombinant vaccines market. The French Medicines Agency (ANSM) has implemented streamlined approval processes for innovative vaccines, which encourages research and development in this sector. In 2025, the agency is expected to expedite the review of recombinant vaccine applications, potentially reducing approval times by up to 30%. This regulatory flexibility is crucial for fostering innovation and ensuring that new vaccines reach the market swiftly. Additionally, the government is actively promoting public-private partnerships to enhance vaccine development initiatives. Such collaborations are likely to strengthen the recombinant vaccines market by facilitating access to funding and resources necessary for research. The supportive regulatory framework is thus a key driver, enabling the rapid advancement of recombinant vaccine technologies in France.
Growing Public Awareness and Acceptance
Public awareness and acceptance of vaccines are crucial factors driving the recombinant vaccines market in France. As health education campaigns become more prevalent, the population is increasingly informed about the benefits of vaccination. In 2025, surveys indicate that approximately 75% of the French population supports vaccination initiatives, reflecting a positive shift in public perception. This growing acceptance is likely to enhance the uptake of recombinant vaccines, as individuals recognize their role in preventing infectious diseases. Furthermore, the influence of social media and community outreach programs is fostering dialogue around vaccine safety and efficacy, which may further bolster public confidence. As a result, the recombinant vaccines market is expected to expand, driven by a well-informed populace that actively seeks vaccination as a means of health protection.
Rising Demand for Preventive Healthcare
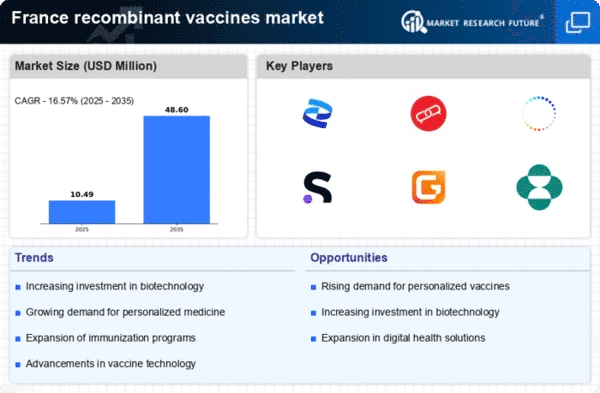
The increasing emphasis on preventive healthcare in France is driving the recombinant vaccines market. As healthcare systems evolve, there is a notable shift towards vaccination as a primary strategy for disease prevention. This trend is underscored by a growing public awareness of health issues and the efficacy of vaccines. In 2025, the French government allocated approximately €500 million to enhance vaccination programs, reflecting a commitment to improving public health. This investment is likely to bolster the recombinant vaccines market, as these vaccines are often at the forefront of innovative preventive measures. Furthermore, the rising incidence of infectious diseases necessitates the development of effective vaccines, thereby creating a robust demand for recombinant technologies. the recombinant vaccines market is poised to benefit from this proactive approach to health management. More individuals seek vaccination as a means to safeguard their health..
Increased Investment in Vaccine Research
Investment in vaccine research is witnessing a notable surge in France, significantly influencing the recombinant vaccines market. In 2025, public and private sectors are projected to invest over €1 billion in vaccine research and development. This influx of capital is aimed at fostering innovation and accelerating the development of new recombinant vaccines. The French government has also introduced tax incentives for companies engaged in vaccine research, further stimulating investment. This financial support is likely to enhance the capabilities of research institutions and biotechnology firms, enabling them to explore novel vaccine candidates. Consequently, the recombinant vaccines market is expected to thrive as a result of this increased investment, leading to the introduction of more effective and diverse vaccine options for the population.
Advancements in Biopharmaceutical Technologies
Technological innovations in biopharmaceuticals are significantly impacting the recombinant vaccines market in France. The development of advanced bioprocessing techniques, such as cell culture and fermentation technologies, has enhanced the production efficiency of recombinant vaccines. In 2025, the market is projected to grow at a CAGR of 8.5%, driven by these advancements. Moreover, the integration of artificial intelligence and machine learning in vaccine development processes is streamlining research and reducing time-to-market for new vaccines. This technological evolution not only improves the quality and safety of vaccines but also lowers production costs, making recombinant vaccines more accessible. As a result, the recombinant vaccines market is likely to expand, catering to the increasing demand for innovative and effective vaccination solutions in France.