Increasing Geriatric Population
The demographic shift towards an aging population in France is significantly impacting the immunosuppressive drugs market. As individuals age, they are more susceptible to various health conditions, including those requiring immunosuppressive therapy, such as organ transplants and autoimmune disorders. Current projections indicate that by 2030, nearly 25% of the French population will be over 65 years old. This demographic trend is likely to drive demand for immunosuppressive drugs, as older adults often require long-term treatment regimens. Consequently, pharmaceutical companies are focusing on developing age-appropriate formulations and delivery systems, which may further enhance market growth in the coming years.
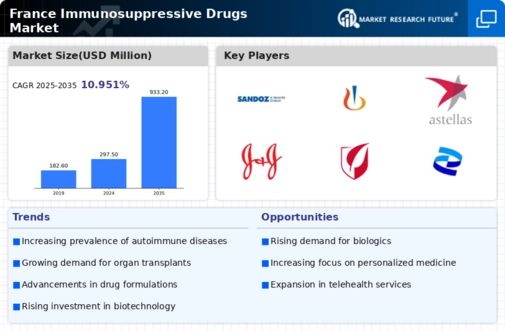
Growing Prevalence of Autoimmune Diseases
The rising incidence of autoimmune diseases in France is a critical driver for the immunosuppressive drugs market. Conditions such as rheumatoid arthritis, lupus, and multiple sclerosis are becoming increasingly common, leading to a heightened demand for effective treatment options. According to recent health statistics, autoimmune diseases affect approximately 5% of the French population, which translates to millions of individuals requiring ongoing medical care. This growing patient base necessitates the development and availability of immunosuppressive therapies, thereby propelling market growth. Furthermore, the increasing awareness and diagnosis of these conditions contribute to the demand for specialized medications, indicating a robust market potential for immunosuppressive drugs in France.
Regulatory Support for Innovative Therapies
The French government has been actively promoting the development of innovative therapies, including immunosuppressive drugs, through favorable regulatory frameworks. Initiatives aimed at expediting the approval process for new medications have been established, which encourages pharmaceutical companies to invest in research and development. The French National Agency for the Safety of Medicines and Health Products (ANSM) plays a pivotal role in this process, ensuring that new immunosuppressive drugs can reach the market efficiently. This regulatory support not only enhances the availability of advanced treatment options but also stimulates competition among manufacturers, potentially leading to lower prices and improved patient access to essential therapies in the immunosuppressive drugs market.
Technological Advancements in Drug Delivery Systems
Innovations in drug delivery systems are transforming the landscape of the immunosuppressive drugs market. Advanced technologies, such as nanotechnology and targeted delivery mechanisms, are being integrated into the development of new immunosuppressive therapies. These advancements aim to improve the efficacy and safety profiles of existing medications, potentially leading to better patient outcomes. In France, research institutions and pharmaceutical companies are collaborating to explore these technologies, which may result in the introduction of more effective immunosuppressive drugs. As these innovations gain traction, they are expected to attract investment and drive growth within the market, reflecting a shift towards more sophisticated treatment options.
Rising Awareness and Education on Immunosuppressive Therapies
There is a growing awareness and education regarding immunosuppressive therapies among healthcare professionals and patients in France. Increased training programs and informational campaigns are being implemented to enhance understanding of the benefits and risks associated with immunosuppressive drugs. This heightened awareness is likely to lead to more informed treatment decisions and increased patient adherence to prescribed therapies. As healthcare providers become more knowledgeable about the latest advancements in immunosuppressive treatments, the demand for these drugs is expected to rise. Consequently, this trend may contribute to the overall growth of the immunosuppressive drugs market, as patients seek effective management options for their conditions.