Surge in R&D Investments
In France, there is a marked increase in research and development (R&D) investments, particularly in the life sciences sector. This surge is largely attributed to government initiatives aimed at fostering innovation and enhancing the competitiveness of the formulation development-outsourcing market. Companies are allocating more resources to R&D to develop novel formulations that meet consumer demands and regulatory requirements. The French government has introduced various funding programs, which have led to a 15% increase in R&D spending among pharmaceutical companies. This trend indicates a growing reliance on outsourcing partners to leverage specialized knowledge and accelerate the formulation development process.
Expansion of Biopharmaceuticals
The biopharmaceutical sector in France is expanding rapidly, significantly impacting the formulation development-outsourcing market. With the increasing prevalence of chronic diseases and the aging population, there is a heightened focus on developing biologics and biosimilars. This expansion is prompting pharmaceutical companies to outsource formulation development to leverage the expertise of specialized firms. The biopharmaceutical market is projected to grow by 12% annually, creating substantial opportunities for outsourcing partners. This trend indicates a shift towards more complex formulations, which require advanced technologies and specialized knowledge that outsourcing firms can provide.
Increasing Regulatory Compliance
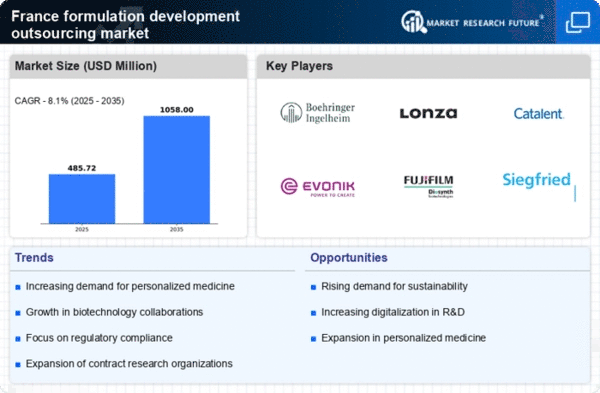
The formulation development-outsourcing market in France is experiencing a notable shift towards stringent regulatory compliance. Regulatory bodies are imposing more rigorous standards for product safety and efficacy, compelling companies to seek external expertise in formulation development. This trend is particularly pronounced in the pharmaceutical and biotechnology sectors, where adherence to regulations can significantly impact market entry and product lifecycle. As a result, outsourcing formulation development has become a strategic approach for companies aiming to navigate complex regulatory landscapes efficiently. The market was projected to grow at a CAGR of approximately 8% over the next five years, driven by the need for compliance with evolving regulations.
Growing Demand for Personalized Medicine
The formulation development-outsourcing market is witnessing a growing demand for personalized medicine in France. As healthcare shifts towards more individualized treatment approaches, pharmaceutical companies are increasingly outsourcing formulation development to create tailored therapies. This trend is driven by advancements in genomics and biotechnology, which enable the development of specific formulations that cater to individual patient needs. The market for personalized medicine is expected to reach €2 billion by 2027, reflecting a compound annual growth rate (CAGR) of 10%. This shift necessitates collaboration with specialized outsourcing partners who possess the expertise to develop complex formulations.
Technological Integration in Formulation Processes
Technological integration is transforming the formulation development-outsourcing market in France. The adoption of advanced technologies such as artificial intelligence, machine learning, and automation is streamlining formulation processes and enhancing efficiency. Companies are increasingly outsourcing to leverage these technologies, which can significantly reduce time-to-market for new products. The integration of technology is expected to improve formulation accuracy and consistency, thereby meeting the high standards of the market. As a result, the formulation development-outsourcing market is anticipated to grow by 9% over the next few years, driven by the need for innovative solutions in formulation development.