Supportive Reimbursement Policies
Supportive reimbursement policies are playing a pivotal role in the growth of the Endometrial Ablation Devices Market. As healthcare systems recognize the cost-effectiveness of these procedures, they are increasingly providing coverage for endometrial ablation treatments. This financial support encourages both patients and healthcare providers to consider these options more seriously. With favorable reimbursement rates, the accessibility of endometrial ablation devices is likely to improve, leading to higher adoption rates. As reimbursement policies continue to evolve, they may further stimulate market growth by making these procedures more financially viable for a larger segment of the population.
Increasing Incidence of Uterine Disorders
The prevalence of uterine disorders, such as fibroids and endometriosis, is contributing to the growth of the Endometrial Ablation Devices Market. As these conditions become more common, the demand for effective treatment options is rising. Statistics indicate that a significant percentage of women experience heavy menstrual bleeding due to these disorders, prompting healthcare providers to seek effective solutions. The ability of endometrial ablation devices to address these issues effectively positions them as a preferred choice among treatment options. Consequently, the market is expected to expand as more women seek relief from the symptoms associated with these conditions.
Rising Awareness of Women's Health Issues
In recent years, there has been a notable increase in awareness surrounding women's health issues, particularly concerning conditions like heavy menstrual bleeding. This heightened awareness is driving demand for effective treatment options, including those offered by the Endometrial Ablation Devices Market. Educational campaigns and advocacy groups are playing a crucial role in informing women about available treatments, which may lead to earlier diagnosis and intervention. As more women seek solutions for their health concerns, the market for endometrial ablation devices is expected to expand, reflecting a growing recognition of the importance of women's health.
Shift Towards Minimally Invasive Procedures
The Endometrial Ablation Devices Market is witnessing a significant shift towards minimally invasive procedures, which are favored for their reduced recovery times and lower complication rates. Patients increasingly prefer treatments that allow them to return to their daily activities quickly. This trend is supported by advancements in surgical techniques and device design, which facilitate outpatient procedures. As healthcare providers adopt these minimally invasive approaches, the market is likely to see a rise in the adoption of endometrial ablation devices. This shift not only enhances patient satisfaction but also aligns with broader trends in healthcare towards less invasive treatment options.
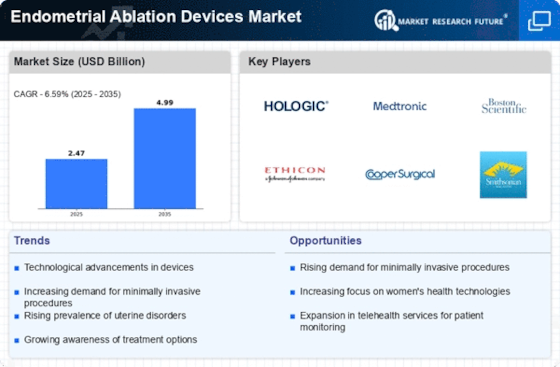
Technological Advancements in Endometrial Ablation Devices
The Endometrial Ablation Devices Market is experiencing a surge in technological advancements that enhance the efficacy and safety of procedures. Innovations such as radiofrequency ablation, cryoablation, and laser ablation are becoming increasingly prevalent. These technologies not only improve patient outcomes but also reduce recovery times, making them more appealing to both patients and healthcare providers. The market is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 6% in the coming years. As these devices become more sophisticated, they are likely to attract a broader patient demographic, thereby expanding the market further.