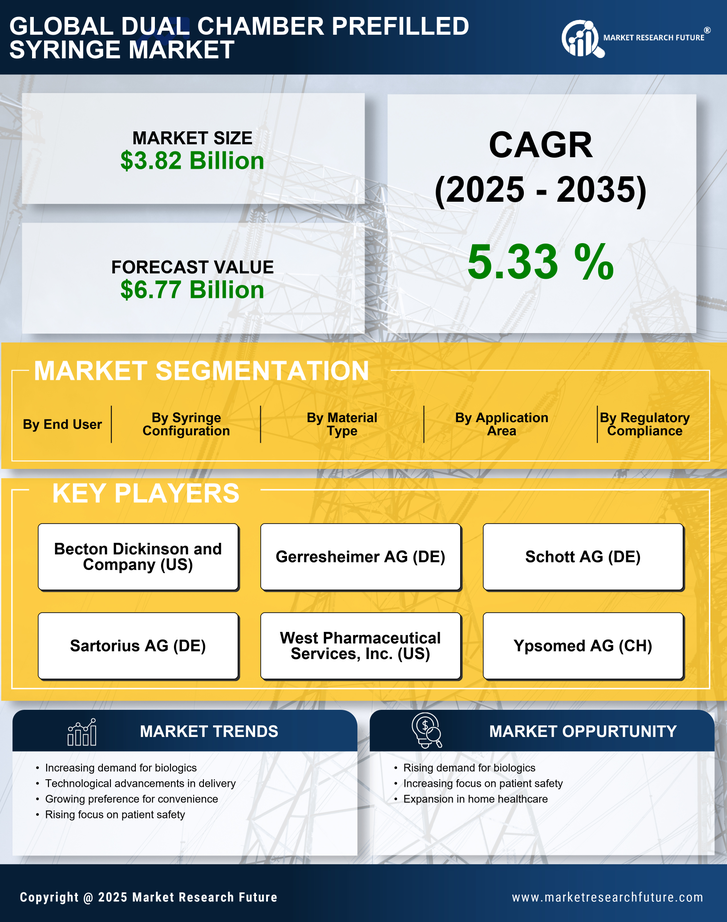
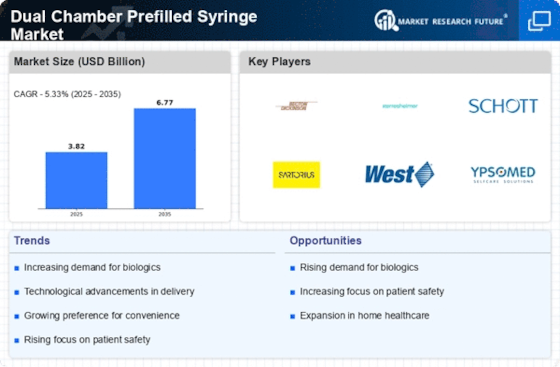
Advancements in Drug Formulation
Innovations in drug formulation are driving growth in the Dual Chamber Prefilled Syringe Market. The ability to combine two incompatible substances in a single device enhances the therapeutic potential of various medications. This is particularly relevant for biologics and complex drugs that require precise dosing and stability. The dual chamber system allows for the mixing of active ingredients just before administration, which can improve patient outcomes and reduce side effects. As pharmaceutical companies continue to invest in research and development, the demand for dual chamber prefilled syringes is expected to rise. Market data indicates that the segment for biologics is expanding rapidly, with dual chamber systems playing a crucial role in delivering these advanced therapies effectively.
Increased Focus on Patient Safety
Patient safety remains a paramount concern in the healthcare sector, significantly influencing the Dual Chamber Prefilled Syringe Market. The design of dual chamber prefilled syringes minimizes the risk of contamination and dosing errors, which are critical factors in ensuring patient safety. As healthcare providers and patients alike prioritize safety, the demand for devices that enhance the reliability of drug delivery systems is likely to increase. Regulatory bodies are also emphasizing the importance of safety features in medical devices, which further propels the adoption of dual chamber prefilled syringes. Market analysis suggests that the emphasis on safety will continue to shape purchasing decisions, leading to a robust growth trajectory for this segment.
Rising Demand for Self-Administration
The increasing trend towards self-administration of medications is a notable driver in the Dual Chamber Prefilled Syringe Market. Patients are increasingly seeking convenient and user-friendly options for medication delivery, particularly for chronic conditions that require regular treatment. This shift is supported by the growing prevalence of diseases such as diabetes and rheumatoid arthritis, which necessitate frequent injections. The dual chamber design allows for the separation of drug components until the point of use, ensuring stability and efficacy. As a result, the market for dual chamber prefilled syringes is projected to expand, with estimates suggesting a compound annual growth rate of over 10% in the coming years. This trend indicates a significant opportunity for manufacturers to innovate and cater to the evolving needs of patients.
Growing Preference for Biologics and Biosimilars
The rising preference for biologics and biosimilars is a significant driver in the Dual Chamber Prefilled Syringe Market. As these complex therapies gain traction, the need for effective delivery systems becomes increasingly critical. Dual chamber prefilled syringes offer a practical solution for administering biologics, allowing for the separation of components until administration, which enhances stability and efficacy. The market for biologics is projected to grow substantially, with estimates indicating a potential increase of over 15% annually. This trend underscores the importance of dual chamber systems in meeting the demands of healthcare providers and patients, thereby positioning the market for continued expansion.
Regulatory Support for Innovative Delivery Systems
Regulatory frameworks are increasingly supportive of innovative drug delivery systems, including the Dual Chamber Prefilled Syringe Market. Authorities are recognizing the benefits of advanced delivery mechanisms that improve patient compliance and therapeutic outcomes. This regulatory backing facilitates faster approvals for new products, encouraging manufacturers to invest in dual chamber technologies. The alignment of regulatory standards with industry needs is likely to foster innovation and expand the market. As a result, the dual chamber prefilled syringe segment is expected to witness a surge in new product launches, driven by both regulatory incentives and market demand for more effective delivery systems.