- Q1 2025: FDA approves Argenica Therapeutics' ARG-007 for Guillain-Barre syndrome clinical trials The U.S. Food and Drug Administration granted approval for Argenica Therapeutics to begin Phase 1 clinical trials of ARG-007, a neuroprotective peptide, in patients with Guillain-Barre syndrome. This marks a significant regulatory milestone for the company in the rare neurological disease space.
- Q2 2024: Octapharma announces new plasma collection center to support immunoglobulin therapies for Guillain-Barre syndrome Octapharma opened a new plasma collection facility in Texas to increase the supply of plasma-derived immunoglobulin products, which are used in the treatment of Guillain-Barre syndrome and other rare neurological disorders.
- Q2 2024: Grifols receives European Commission approval for new intravenous immunoglobulin formulation Grifols announced that the European Commission has approved its new formulation of intravenous immunoglobulin (IVIG), expanding treatment options for patients with Guillain-Barre syndrome and other immune-mediated neuropathies.
- Q3 2024: CSL Behring launches Hizentra® prefilled syringes for chronic inflammatory demyelinating polyneuropathy and Guillain-Barre syndrome CSL Behring introduced prefilled syringes of Hizentra®, an immunoglobulin therapy, in the U.S. market, aiming to improve administration convenience for patients with Guillain-Barre syndrome and related conditions.
- Q1 2025: Geneius Biotherapeutics raises $40M Series B to advance gene therapy for Guillain-Barre syndrome Geneius Biotherapeutics secured $40 million in Series B funding to accelerate the development of its investigational gene therapy targeting Guillain-Barre syndrome, with plans to initiate clinical trials in late 2025.
- Q2 2024: Takeda and NeuroNext announce partnership to develop novel therapies for Guillain-Barre syndrome Takeda and NeuroNext entered a strategic partnership to co-develop and commercialize new therapeutic candidates for Guillain-Barre syndrome, leveraging NeuroNext's platform for rapid clinical trial execution.
- Q3 2024: FDA grants orphan drug designation to ImmunoGenix's IGX-101 for Guillain-Barre syndrome ImmunoGenix received orphan drug designation from the U.S. FDA for IGX-101, its lead candidate for the treatment of Guillain-Barre syndrome, providing regulatory incentives for further development.
- Q2 2025: BioNTech initiates Phase 2 trial of mRNA-based therapy for Guillain-Barre syndrome BioNTech announced the start of a Phase 2 clinical trial evaluating its mRNA-based therapeutic candidate in patients with Guillain-Barre syndrome, marking the company's expansion into rare neurological diseases.
- Q1 2024: Natus Medical launches next-generation EMG system for Guillain-Barre syndrome diagnostics Natus Medical introduced a new electromyography (EMG) system designed to enhance the diagnosis of Guillain-Barre syndrome, featuring improved sensitivity and workflow integration for neurology clinics.
- Q4 2024: Medtronic appoints Dr. Lisa Chen as Head of Neurology Division, overseeing Guillain-Barre syndrome portfolio Medtronic named Dr. Lisa Chen as the new Head of its Neurology Division, where she will lead the company's strategic initiatives in the Guillain-Barre syndrome treatment and diagnostics market.
- Q2 2025: Sanofi acquires NeuroVax for $350 million to expand Guillain-Barre syndrome pipeline Sanofi completed the acquisition of NeuroVax, a biotech firm specializing in autoimmune neurology, to strengthen its pipeline of therapies for Guillain-Barre syndrome and related disorders.
- Q3 2025: FDA approves expanded indication for Octagam® 10% in treatment of Guillain-Barre syndrome The U.S. FDA approved an expanded indication for Octagam® 10%, an intravenous immunoglobulin product from Octapharma, allowing its use in the treatment of Guillain-Barre syndrome in adult patients.
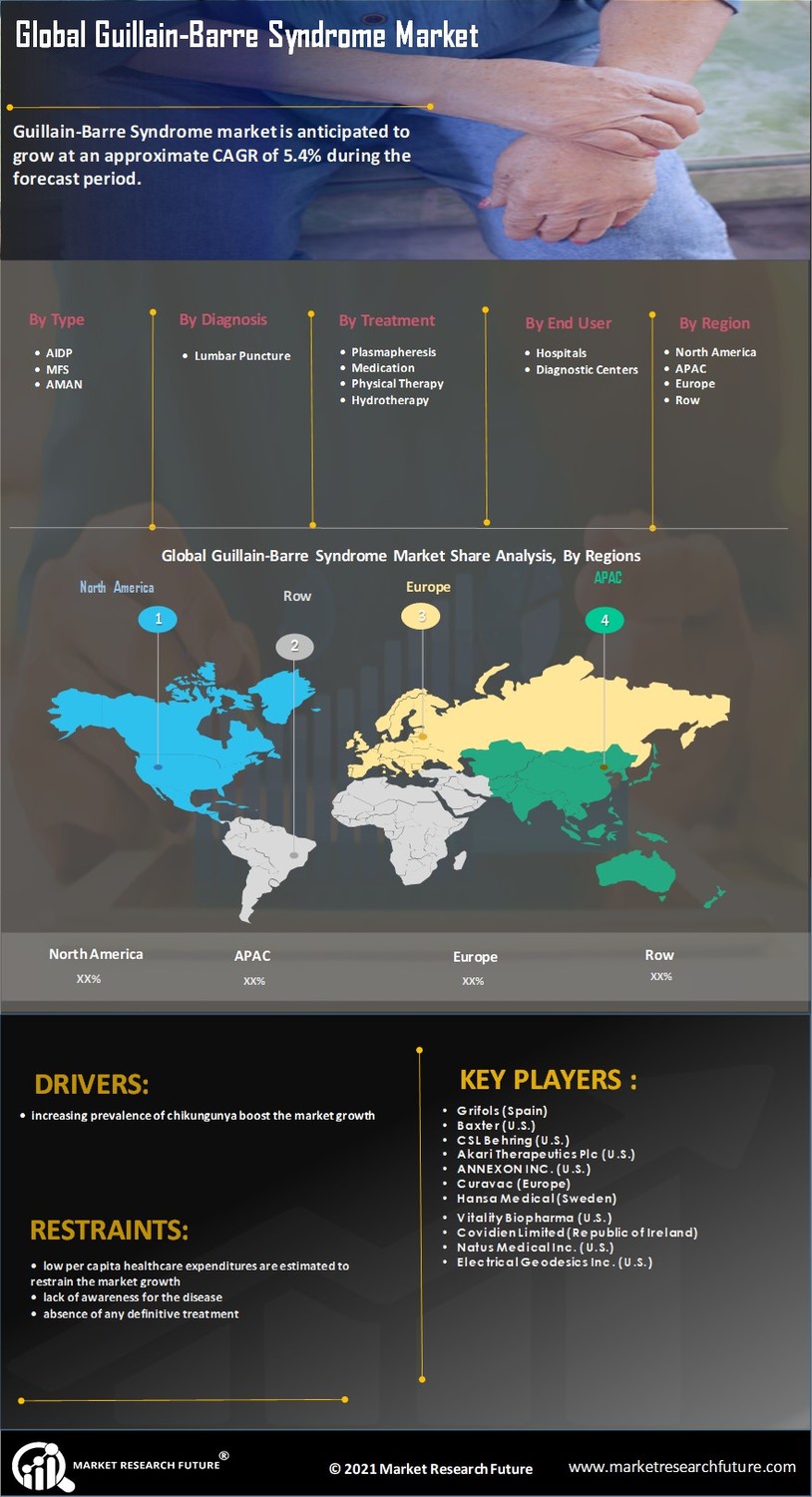
Guillain-Barre Syndrome Market Segment Insights
The Guillain-Barre Syndrome Market is segmented on the basis of type, diagnosis, treatment, and end-user.
Guillain-Barre Syndrome Market Type Insights
On the basis of type, the Guillain-Barre Syndrome Market is segmented into Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP), Miller Fisher Syndrome (MFS), Acute Motor Axonal Neuropathy (AMAN), and others.
Guillain-Barre Syndrome Market Diagnosis Insights
On the basis of diagnosis, the market is categorized into lumbar puncture, electromyography, nerve conduction studies, and others. The electromyography segment is sub-segmented into intramuscular electromyography, surface electromyography, and others.
Guillain-Barre Syndrome Market Treatment Insights
On the basis of treatment, the market is segmented into plasmapheresis, medication, physical therapy, hydrotherapy, and others. The medication segment is sub-segmented into privigen intravenous, gammagard liquid injection, flebogamma DIF intravenous, bivigam intravenous, and others.
Guillain-Barre Syndrome Market End-User Insights
On the basis of end-user, the market is segmented into hospitals and clinics, diagnostic centers, and others.
Guillain-Barre Syndrome Market Regional Insights
America dominates the Guillain-Barre Syndrome Market in the presence of a well-developed healthcare sector and huge patient population. Moreover, the changing lifestyle and rising per capita healthcare expenditure fuel the market. Additionally, the presence of developed economies such as the U.S. and Canada within the region fuels the market growth.
Europe stands second in the Guillain-Barre Syndrome Market. This can be attributed on the basis of availability of funds for research and a huge patient population. On a regional basis, Europe is segmented into Western Europe and Eastern Europe. Western Europe is estimated to lead the market while Eastern Europe is estimated to be the fastest growing region.
Asia Pacific is projected to be the fastest growing region for the Guillain-Barre syndrome market due to the presence of huge patient population and developing economies such as India and China within the region. Moreover, a growing healthcare sector and the growing government support boost the regional market for the Asia Pacific region.
On the other hand, the Middle East and Africa holds the least share of the global Guillain-Barre Syndrome Market. This can be attributed to the presence of poor economies, lack of healthcare services, and stringent government policies, especially in the African region. A majority of the regions of the Middle East and Africa is held by the Middle East owing to the increasing per capita healthcare expenditure by developed economies such as Dubai, Kuwait, and Saudi Arabia within the region.
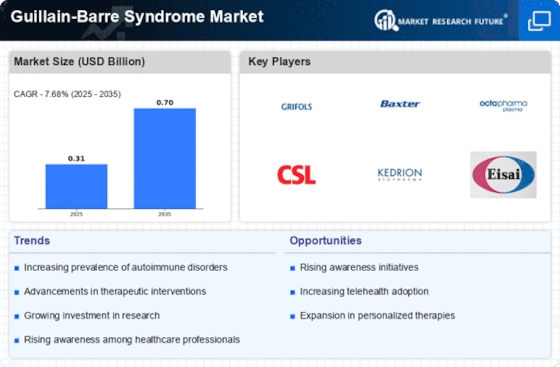
Key Players in the Guillain-Barre Syndrome Market
The key players in the Guillain-Barre Syndrome Market are
- Grifols (Spain)
- Baxter (U.S.)
- CSL Behring (U.S.)
- Akari Therapeutics Plc (U.S.)
- ANNEXON, INC. (U.S.)
- Curavac (Europe)
- Hansa Medical (Sweden)
- Vitality Biopharma (U.S.)
- Covidien Limited (Republic of Ireland)
- Natus Medical Inc. (U.S.)
- Electrical Geodesics Inc. (U.S.)
Intended Audience
- Pharmaceutical Companies
- Biotechnological Institutes
- Government and Private Laboratories
- Research and Development (R&D) Companies
- Medical Research Laboratories
- Market Research and Consulting Service Providers