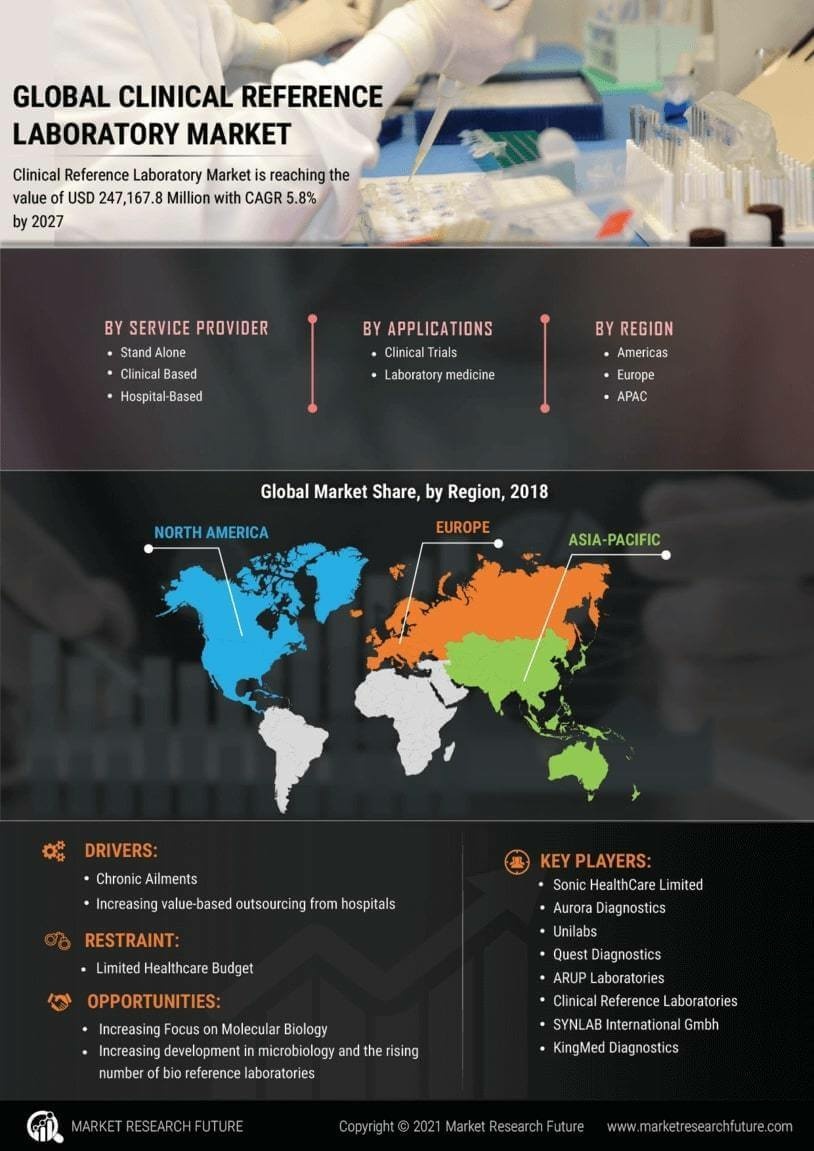
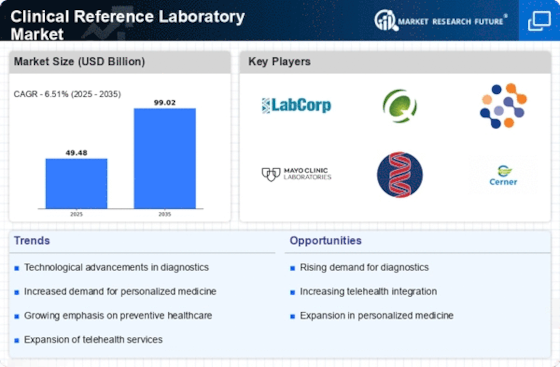
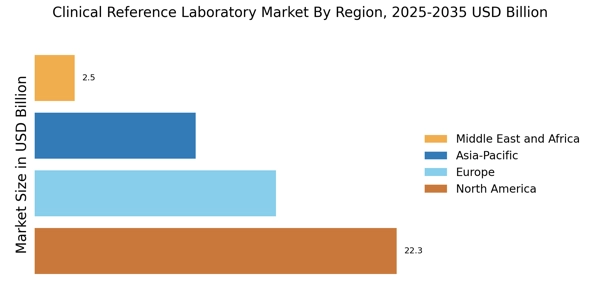
Rising Demand for Diagnostic Testing
The Clinical Reference Laboratory Market is experiencing a notable increase in demand for diagnostic testing services. This surge is primarily driven by the growing prevalence of chronic diseases and the aging population, which necessitates regular health monitoring. According to recent statistics, the demand for laboratory tests is projected to grow at a compound annual growth rate of approximately 7% over the next few years. This trend indicates that healthcare providers are increasingly relying on clinical reference laboratories to deliver accurate and timely test results, thereby enhancing patient care. Furthermore, advancements in laboratory technologies are enabling faster turnaround times, which is likely to further boost the demand for these services. As a result, clinical reference laboratories are positioned to play a crucial role in the evolving healthcare landscape.
Advancements in Laboratory Technology
Technological innovations are significantly transforming the Clinical Reference Laboratory Market. The integration of automation, artificial intelligence, and advanced diagnostic tools is enhancing the efficiency and accuracy of laboratory operations. For instance, the adoption of high-throughput screening technologies allows laboratories to process a larger volume of tests in a shorter timeframe. This is particularly relevant as the market anticipates a growth trajectory, with estimates suggesting a market value exceeding 50 billion dollars by 2026. Moreover, the implementation of telemedicine and remote diagnostics is facilitating access to laboratory services, especially in underserved regions. These advancements not only improve operational efficiency but also contribute to better patient outcomes, thereby solidifying the role of clinical reference laboratories in modern healthcare.
Increased Focus on Preventive Healthcare
The Clinical Reference Laboratory Market is witnessing a paradigm shift towards preventive healthcare. This shift is largely influenced by rising healthcare costs and a growing awareness of the importance of early disease detection. Preventive testing is becoming a standard practice, with more individuals opting for routine screenings to identify potential health issues before they escalate. This trend is reflected in the increasing volume of tests conducted by clinical reference laboratories, which is expected to rise significantly in the coming years. Additionally, healthcare policies are increasingly supporting preventive measures, further driving the demand for laboratory services. As a result, clinical reference laboratories are likely to expand their offerings to include a wider range of preventive tests, thereby enhancing their market presence.
Regulatory Changes and Compliance Requirements
The Clinical Reference Laboratory Market is heavily influenced by regulatory changes and compliance requirements. Governments and health authorities are continuously updating regulations to ensure the quality and safety of laboratory services. These regulations often necessitate clinical reference laboratories to invest in quality management systems and adhere to stringent operational standards. Compliance with these regulations not only enhances the credibility of laboratories but also fosters trust among healthcare providers and patients. As the regulatory landscape evolves, laboratories that proactively adapt to these changes are likely to gain a competitive advantage. Furthermore, the emphasis on accreditation and certification is expected to drive the growth of the clinical reference laboratory market, as laboratories strive to meet the highest standards of service delivery.
Growing Collaboration with Healthcare Providers
Collaboration between clinical reference laboratories and healthcare providers is becoming increasingly prevalent in the Clinical Reference Laboratory Market. Such partnerships are essential for streamlining laboratory services and improving patient care. By working closely with hospitals, clinics, and physicians, laboratories can better understand the specific testing needs of their clients and tailor their services accordingly. This collaborative approach is likely to enhance the efficiency of diagnostic processes and ensure timely delivery of results. Additionally, these partnerships may facilitate the development of innovative testing solutions that address emerging health challenges. As the healthcare landscape continues to evolve, the synergy between clinical reference laboratories and healthcare providers is expected to play a pivotal role in shaping the future of laboratory services.