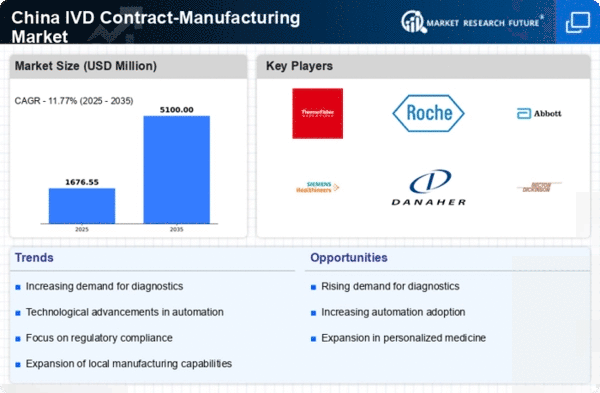
Rising Demand for Diagnostic Testing
The increasing prevalence of chronic diseases and the aging population in China are driving the demand for diagnostic testing. This trend is expected to bolster the ivd contract-manufacturing market as healthcare providers seek efficient and reliable testing solutions. According to recent estimates, the diagnostic testing market in China is projected to grow at a CAGR of approximately 10% over the next five years. This growth is likely to create opportunities for contract manufacturers to expand their services and capabilities, catering to the rising needs of healthcare facilities. As hospitals and laboratories look to streamline operations, the reliance on contract manufacturing for in vitro diagnostics is anticipated to increase, thereby enhancing the overall market landscape.
Expansion of Healthcare Infrastructure
The expansion of healthcare infrastructure in China is a pivotal driver for the ivd contract-manufacturing market. With the government's commitment to improving healthcare access and quality, there is a notable increase in the establishment of hospitals and diagnostic centers. This expansion is expected to create a higher demand for in vitro diagnostic products, thereby stimulating the need for contract manufacturing services. As new healthcare facilities emerge, the requirement for reliable and efficient diagnostic solutions will likely rise, presenting opportunities for contract manufacturers to cater to this growing market. The overall investment in healthcare infrastructure is projected to reach several billion dollars in the coming years, further solidifying the role of contract manufacturing in the ivd sector.
Cost Efficiency and Resource Optimization
In the context of the ivd contract-manufacturing market, cost efficiency remains a critical driver. Many companies in China are opting for contract manufacturing to reduce operational costs and optimize resource allocation. By outsourcing production, firms can focus on core competencies such as research and development while leveraging the expertise of specialized manufacturers. This approach not only minimizes capital expenditure but also allows for scalability in production. Reports indicate that companies can save up to 30% in manufacturing costs by utilizing contract services. As the competitive landscape intensifies, the pursuit of cost-effective solutions is likely to propel the growth of the ivd contract-manufacturing market.
Regulatory Compliance and Quality Assurance
Regulatory compliance is a significant driver for the ivd contract-manufacturing market in China. The stringent regulations imposed by health authorities necessitate that manufacturers adhere to high standards of quality and safety. Companies that engage in contract manufacturing must ensure that their processes align with these regulations to avoid penalties and maintain market access. This focus on compliance not only enhances product quality but also builds trust with healthcare providers and patients. As the regulatory landscape continues to evolve, manufacturers that prioritize compliance are likely to gain a competitive edge in the ivd contract-manufacturing market.
Technological Integration in Manufacturing Processes
The integration of advanced technologies such as automation and artificial intelligence in manufacturing processes is transforming the ivd contract-manufacturing market. In China, manufacturers are increasingly adopting these technologies to enhance production efficiency and ensure high-quality output. The implementation of automated systems can reduce human error and increase throughput, which is essential in meeting the growing demand for diagnostic products. Furthermore, the use of AI in quality control processes can lead to improved accuracy and reliability in testing. As these technologies become more prevalent, they are expected to drive innovation and competitiveness within the ivd contract-manufacturing market.