Regulatory Support and Frameworks
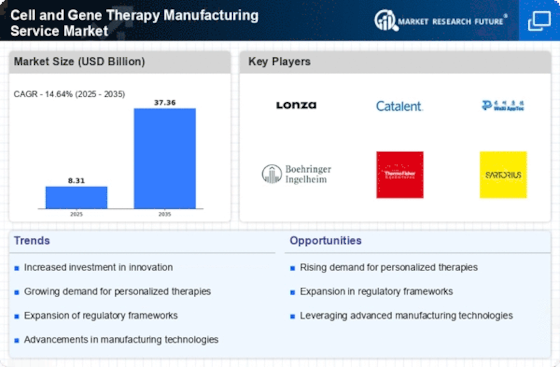
The establishment of supportive regulatory frameworks is crucial for the Cell and Gene Therapy Manufacturing Service Market. Regulatory bodies are increasingly recognizing the potential of gene and cell therapies, leading to expedited approval processes and guidelines that facilitate innovation. For instance, the introduction of programs like the FDA's Breakthrough Therapy Designation has accelerated the development of promising therapies. This regulatory support encourages investment in manufacturing capabilities, as companies seek to navigate the complex landscape of compliance while bringing novel therapies to market. As a result, the Cell and Gene Therapy Manufacturing Service Market is likely to experience robust growth driven by these favorable regulatory conditions.
Rising Prevalence of Genetic Disorders
The increasing incidence of genetic disorders is a pivotal driver for the Cell and Gene Therapy Manufacturing Service Market. As more individuals are diagnosed with conditions such as hemophilia, muscular dystrophy, and various inherited diseases, the demand for innovative therapies rises. According to recent estimates, genetic disorders affect approximately 1 in 10 individuals, underscoring the urgent need for effective treatments. This growing patient population propels investments in research and development, leading to a surge in the production of gene therapies. Consequently, manufacturers are compelled to enhance their capabilities to meet the escalating demand for tailored therapeutic solutions, thereby driving growth in the Cell and Gene Therapy Manufacturing Service Market.
Increased Investment in Biopharmaceuticals
The surge in investment within the biopharmaceutical sector significantly influences the Cell and Gene Therapy Manufacturing Service Market. Venture capital and private equity funding have seen remarkable increases, with billions allocated to companies focused on gene and cell therapies. This influx of capital not only supports research and development but also enhances manufacturing capabilities, allowing for the scaling of production processes. As biopharmaceutical companies expand their portfolios to include gene therapies, the demand for specialized manufacturing services rises. This trend indicates a promising trajectory for the Cell and Gene Therapy Manufacturing Service Market, as financial backing fuels innovation and operational expansion.
Technological Advancements in Gene Editing
Technological innovations, particularly in gene editing techniques like CRISPR and TALEN, are transforming the landscape of the Cell and Gene Therapy Manufacturing Service Market. These advancements enable precise modifications to genetic material, facilitating the development of targeted therapies. The global market for gene editing technologies is projected to reach substantial figures, reflecting the increasing adoption of these methods in therapeutic applications. As researchers and manufacturers harness these technologies, the efficiency and effectiveness of gene therapies improve, leading to a broader acceptance and integration into clinical practice. This trend not only enhances the therapeutic potential but also stimulates the growth of the Cell and Gene Therapy Manufacturing Service Market.
Growing Patient Demand for Innovative Therapies
The rising patient demand for innovative therapies is a driving force in the Cell and Gene Therapy Manufacturing Service Market. Patients increasingly seek advanced treatment options that offer potential cures for previously untreatable conditions. This shift in patient expectations is prompting healthcare providers to explore and adopt gene therapies, which are often seen as a last resort for severe diseases. Market Research Future indicates that the willingness to pay for these therapies is high, reflecting the perceived value of innovative solutions. As patient advocacy groups and healthcare professionals promote awareness of gene therapies, the demand for manufacturing services tailored to these products is likely to escalate, further propelling the growth of the Cell and Gene Therapy Manufacturing Service Market.