Rising Incidence of B-cell Malignancies
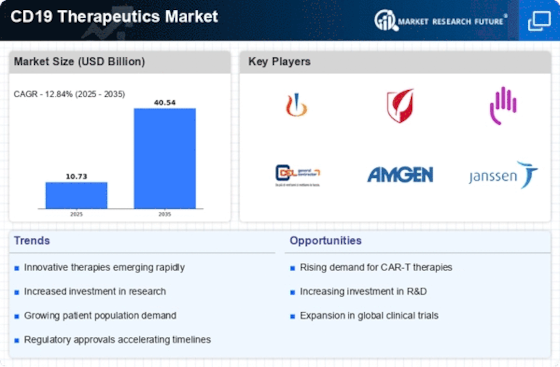
The increasing prevalence of B-cell malignancies, such as non-Hodgkin lymphoma and chronic lymphocytic leukemia, is a primary driver of the CD19 Therapeutics Market. According to recent data, the incidence of these cancers has been on the rise, with estimates suggesting that over 200,000 new cases are diagnosed annually. This growing patient population necessitates innovative treatment options, thereby propelling the demand for CD19-targeted therapies. As healthcare systems strive to improve patient outcomes, the focus on effective and targeted therapies becomes paramount. The CD19 Therapeutics Market is likely to benefit from this trend, as healthcare providers seek to adopt advanced treatment modalities that offer better efficacy and safety profiles. Consequently, the market is expected to expand significantly in response to the increasing burden of B-cell malignancies.
Increased Investment in Oncology Research
The surge in investment directed towards oncology research is a significant driver of the CD19 Therapeutics Market. Pharmaceutical companies and research institutions are allocating substantial resources to develop novel therapies targeting B-cell malignancies. Recent reports indicate that global funding for cancer research has reached unprecedented levels, with billions of dollars being invested annually. This influx of capital is facilitating the exploration of innovative treatment modalities, including CD19-targeted therapies. As a result, the CD19 Therapeutics Market is likely to experience accelerated growth, as new therapies emerge from the research pipeline. Furthermore, collaborations between academia and industry are fostering a conducive environment for the development of cutting-edge therapies, thereby enhancing the overall market landscape.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development and approval of innovative therapies, which is positively impacting the CD19 Therapeutics Market. Initiatives aimed at expediting the review process for breakthrough therapies are encouraging pharmaceutical companies to invest in CD19-targeted treatments. Recent regulatory approvals for CAR-T therapies have set a precedent, demonstrating the willingness of authorities to facilitate access to potentially life-saving treatments. This supportive regulatory environment is likely to enhance the attractiveness of the CD19 Therapeutics Market for investors and developers alike. As more therapies receive expedited approval, the market is expected to expand, driven by the increasing availability of effective treatment options for patients suffering from B-cell malignancies.
Technological Advancements in CAR-T Cell Therapy
Technological innovations in CAR-T cell therapy are transforming the landscape of the CD19 Therapeutics Market. Recent advancements in gene editing and cell manufacturing processes have enhanced the efficacy and safety of CAR-T therapies. For instance, the development of next-generation CAR-T cells, which exhibit improved persistence and reduced toxicity, is likely to attract more patients and healthcare providers. Market data indicates that the CAR-T cell therapy segment is projected to grow at a compound annual growth rate of over 30% in the coming years. This surge is attributed to the increasing approval of novel CAR-T products targeting CD19, which are demonstrating promising clinical outcomes. As these technologies continue to evolve, the CD19 Therapeutics Market is expected to witness substantial growth, driven by the demand for more effective and accessible treatment options.
Growing Awareness and Education on B-cell Malignancies
The rising awareness and education surrounding B-cell malignancies are contributing to the growth of the CD19 Therapeutics Market. Increased public and professional knowledge about these diseases is leading to earlier diagnosis and treatment, which is crucial for improving patient outcomes. Educational campaigns and initiatives by healthcare organizations are playing a pivotal role in disseminating information about the importance of targeted therapies, including those that target CD19. As awareness grows, more patients are likely to seek treatment options, thereby driving demand for CD19-targeted therapies. Market data suggests that this trend is expected to continue, as healthcare providers increasingly recognize the benefits of early intervention and the role of innovative therapies in managing B-cell malignancies.