Rising Incidence of Cancer
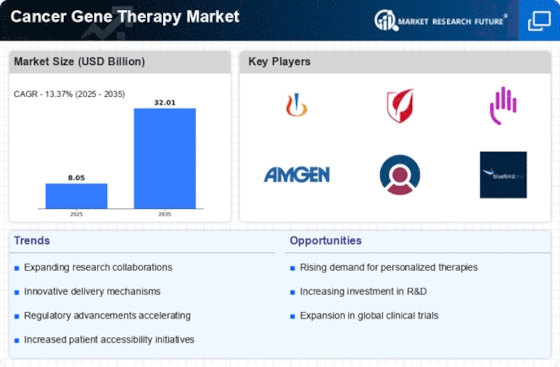
The increasing prevalence of cancer worldwide is a primary driver for the Cancer Gene Therapy Market. According to recent statistics, cancer cases are projected to rise significantly, with estimates suggesting that by 2040, the number of new cancer cases could reach over 27 million annually. This alarming trend necessitates innovative treatment options, including gene therapy, which aims to target the genetic basis of cancer. As healthcare systems grapple with the growing burden of cancer, the demand for effective therapies is likely to escalate, propelling the Cancer Gene Therapy Market forward. Furthermore, the shift towards more personalized treatment approaches aligns with the capabilities of gene therapy, which can be tailored to individual genetic profiles, thereby enhancing treatment efficacy and patient outcomes.
Increased Investment in Cancer Research
The Cancer Gene Therapy Market is experiencing a surge in investment from both public and private sectors. Governments and organizations are recognizing the urgent need for innovative cancer treatments, leading to increased funding for research initiatives. For instance, funding for cancer research has seen a substantial rise, with billions allocated annually to support clinical trials and the development of new therapies. This influx of capital is fostering collaboration among researchers, biotech companies, and academic institutions, which is essential for advancing gene therapy technologies. As more resources are dedicated to understanding cancer at the molecular level, the potential for breakthroughs in gene therapy is likely to expand, further propelling the Cancer Gene Therapy Market.
Advancements in Gene Editing Technologies
Recent advancements in gene editing technologies, such as CRISPR and TALEN, are revolutionizing the Cancer Gene Therapy Market. These technologies enable precise modifications to the genetic material of cancer cells, offering new avenues for treatment. The ability to edit genes with high accuracy has led to the development of novel therapies that can potentially cure previously untreatable cancers. Market data indicates that the gene editing market is expected to grow at a compound annual growth rate of over 30% in the coming years, reflecting the increasing investment in research and development. As these technologies continue to evolve, they are likely to enhance the effectiveness and safety of gene therapies, thereby driving growth in the Cancer Gene Therapy Market.
Regulatory Support for Gene Therapy Approvals
Regulatory bodies are increasingly supportive of gene therapy innovations, which is a crucial driver for the Cancer Gene Therapy Market. Recent regulatory frameworks have been established to expedite the approval process for gene therapies, recognizing their potential to address unmet medical needs in oncology. For example, the introduction of breakthrough therapy designations allows for faster review and approval of promising gene therapies. This regulatory support not only encourages pharmaceutical companies to invest in gene therapy research but also instills confidence in investors and stakeholders. As more gene therapies receive regulatory approval, the market is likely to witness a surge in available treatment options, further stimulating growth in the Cancer Gene Therapy Market.
Growing Awareness and Acceptance of Gene Therapies
There is a notable increase in awareness and acceptance of gene therapies among healthcare professionals and patients, which is positively impacting the Cancer Gene Therapy Market. Educational campaigns and successful case studies are helping to demystify gene therapy, showcasing its potential benefits in treating various cancers. As patients become more informed about their treatment options, they are increasingly advocating for advanced therapies, including gene therapy. Market Research Future indicates that patient acceptance of gene therapies is on the rise, with many expressing willingness to consider these innovative treatments. This shift in perception is likely to drive demand for gene therapy solutions, thereby contributing to the growth of the Cancer Gene Therapy Market.