Growing Awareness and Advocacy
The increasing awareness and advocacy for Canavan disease are driving the Canavan Disease Treatment Market. Non-profit organizations and patient advocacy groups are playing a vital role in educating the public and healthcare professionals about the disease. Their efforts are leading to earlier diagnoses and a greater demand for effective treatments. Additionally, awareness campaigns are encouraging research funding and collaboration among stakeholders, including researchers, clinicians, and pharmaceutical companies. As awareness continues to grow, the Canavan Disease Treatment Market is likely to benefit from increased interest in developing new therapies and improving patient care, ultimately enhancing the overall landscape of treatment options.
Advancements in Genetic Research
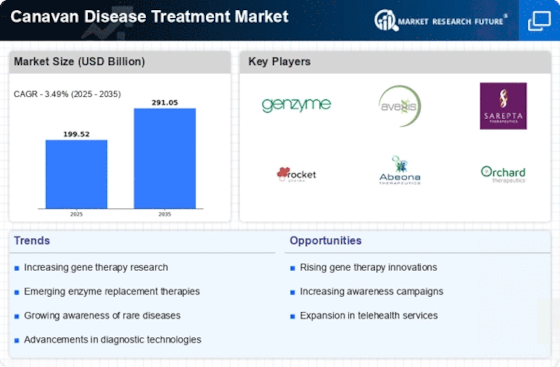
Recent advancements in genetic research are significantly influencing the Canavan Disease Treatment Market. The identification of the ASPA gene mutation, responsible for Canavan disease, has opened avenues for targeted therapies, including gene therapy and enzyme replacement strategies. These innovations are expected to enhance treatment efficacy and patient outcomes. Furthermore, the global market for gene therapy is projected to reach USD 10 billion by 2025, indicating a robust interest in genetic-based treatments. As research continues to evolve, the Canavan Disease Treatment Market may witness the introduction of novel therapies that could transform the management of this condition, ultimately improving the quality of life for affected individuals.
Regulatory Support for Orphan Drugs
Regulatory frameworks supporting orphan drugs are significantly impacting the Canavan Disease Treatment Market. Governments worldwide are implementing policies that incentivize the development of treatments for rare diseases, including Canavan disease. These incentives often include tax breaks, extended market exclusivity, and expedited review processes. For instance, the Orphan Drug Act in the United States has facilitated the approval of numerous therapies for rare conditions. Such regulatory support not only encourages pharmaceutical companies to invest in the Canavan Disease Treatment Market but also accelerates the availability of new treatments for patients. This environment fosters innovation and could lead to a more diverse range of therapeutic options.
Rising Prevalence of Canavan Disease
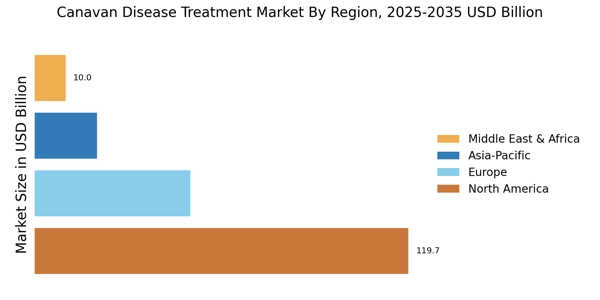
The increasing incidence of Canavan disease is a pivotal driver for the Canavan Disease Treatment Market. As awareness of genetic disorders grows, more cases are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that Canavan disease affects approximately 1 in 40,000 births, particularly among individuals of Ashkenazi Jewish descent. This demographic concentration indicates a potential market segment that could benefit from targeted therapies. The rising prevalence not only emphasizes the need for innovative treatments but also encourages investment in research and development. Pharmaceutical companies are likely to respond to this demand by developing new therapies, thereby expanding the Canavan Disease Treatment Market.
Increased Investment in Rare Disease Research
The growing investment in research for rare diseases is a crucial driver for the Canavan Disease Treatment Market. Governments and private organizations are increasingly allocating funds to develop therapies for conditions like Canavan disease, which have historically been overlooked. In recent years, funding for rare disease research has surged, with estimates indicating that over USD 1 billion is invested annually in the United States alone. This financial support fosters innovation and encourages pharmaceutical companies to explore treatment options for Canavan disease. As a result, the Canavan Disease Treatment Market is likely to expand, with new therapies emerging to address the unmet medical needs of patients.