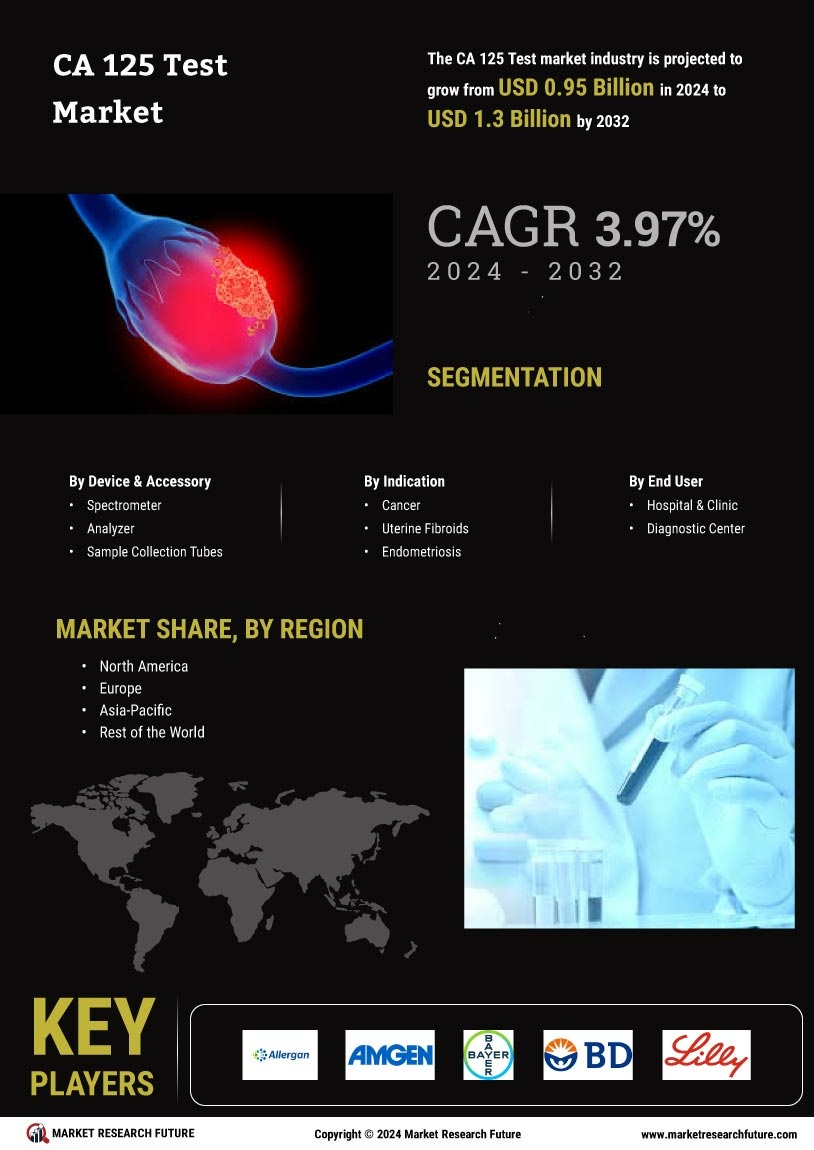
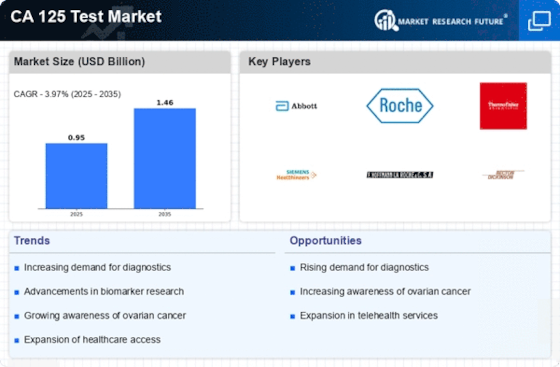
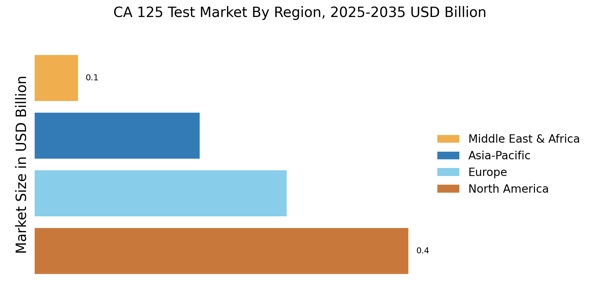
Growing Focus on Women's Health
The CA 125 Test Market is benefiting from the increasing emphasis on women's health issues. As healthcare systems worldwide prioritize women's health, there is a corresponding rise in the availability of diagnostic tests specifically designed for conditions affecting women, such as ovarian cancer. This focus is reflected in the growing number of awareness campaigns and educational programs aimed at informing women about the importance of early detection. Consequently, more women are seeking CA 125 testing as part of their routine health check-ups. The market is likely to see sustained growth as healthcare providers continue to advocate for comprehensive women's health services, thereby enhancing the visibility and accessibility of the CA 125 test.
Rising Incidence of Ovarian Cancer
The CA 125 Test Market is experiencing growth due to the rising incidence of ovarian cancer. According to recent statistics, ovarian cancer cases have been steadily increasing, leading to heightened demand for effective diagnostic tools. The CA 125 test, which measures the level of the CA 125 protein in the blood, is crucial for monitoring treatment response and detecting recurrence. As awareness of ovarian cancer symptoms grows, more individuals are seeking testing, thereby driving market expansion. Furthermore, the increasing focus on early detection and personalized treatment options is likely to further propel the CA 125 Test Market. This trend suggests that healthcare providers are prioritizing the implementation of advanced diagnostic tests to improve patient outcomes.
Advancements in Diagnostic Technologies
Technological innovations are significantly influencing the CA 125 Test Market. The development of more sensitive and specific assays for measuring CA 125 levels has enhanced the accuracy of ovarian cancer diagnosis. These advancements not only improve patient outcomes but also increase the reliability of the test results. The integration of automation and artificial intelligence in laboratory settings is streamlining testing processes, thereby reducing turnaround times. As a result, healthcare facilities are more inclined to adopt these advanced diagnostic technologies, which is likely to boost the CA 125 Test Market. Moreover, the ongoing research into novel biomarkers may complement the CA 125 test, further expanding its application in clinical settings.
Increased Investment in Cancer Research
The CA 125 Test Market is experiencing growth due to increased investment in cancer research. Funding from both public and private sectors is being directed towards understanding ovarian cancer and improving diagnostic methods. This influx of capital is facilitating the development of novel testing methodologies and enhancing existing CA 125 tests. Research initiatives are focusing on identifying new biomarkers that could complement CA 125, potentially leading to more accurate diagnostic tools. As research progresses, the CA 125 Test Market is likely to benefit from the introduction of innovative products that meet the evolving needs of healthcare providers and patients alike. This trend indicates a promising future for the market as advancements in cancer research continue to unfold.
Regulatory Support for Diagnostic Testing
The CA 125 Test Market is positively impacted by supportive regulatory frameworks that facilitate the approval and use of diagnostic tests. Regulatory agencies are increasingly recognizing the importance of early detection in improving cancer outcomes, leading to streamlined approval processes for innovative diagnostic tools. This regulatory support encourages manufacturers to invest in research and development, resulting in the introduction of new and improved CA 125 testing methods. As a consequence, the market is likely to expand as healthcare providers gain access to a wider range of reliable diagnostic options. Furthermore, favorable reimbursement policies for CA 125 tests may enhance their adoption in clinical practice, further driving the growth of the CA 125 Test Market.