Stringent Regulatory Frameworks
The Biologic Excipient Market is shaped by stringent regulatory frameworks that govern the development and approval of biologics. Regulatory agencies are increasingly emphasizing the quality and safety of excipients used in biologic formulations. This heightened scrutiny is likely to drive manufacturers to invest in high-quality excipients that comply with regulatory standards. In 2025, the market for regulatory-compliant excipients is expected to grow, reflecting the industry's commitment to safety and efficacy. As companies navigate these regulations, the demand for biologic excipients that meet rigorous quality standards will likely increase, thereby influencing market dynamics. This trend underscores the importance of regulatory compliance in shaping the future of the biologic excipient market.
Increasing Investment in Biologics
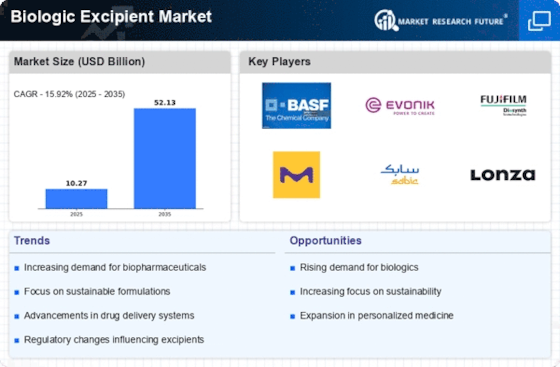
The Biologic Excipient Market is experiencing a surge in investment as pharmaceutical companies recognize the potential of biologics. This trend is driven by the increasing prevalence of chronic diseases and the need for innovative therapies. In 2025, the market for biologics is projected to reach approximately 300 billion USD, indicating a robust growth trajectory. As companies allocate more resources towards research and development, the demand for biologic excipients is likely to rise. These excipients play a crucial role in enhancing the stability and efficacy of biologic formulations, thereby attracting significant attention from investors. The influx of capital is expected to foster innovation and expand the range of available biologic excipients, ultimately benefiting the market.
Growing Focus on Sustainable Practices
The Biologic Excipient Market is increasingly influenced by a growing focus on sustainability. As environmental concerns gain prominence, pharmaceutical companies are seeking excipients that are derived from renewable sources and have a lower environmental impact. This shift towards sustainable practices is likely to drive innovation in the development of biologic excipients. In 2025, the market for sustainable excipients is projected to expand significantly, reflecting the industry's response to consumer demand for environmentally friendly products. Manufacturers are encouraged to explore biodegradable and eco-friendly excipients, which could enhance their competitive edge in the biologic excipient market. This trend not only aligns with global sustainability goals but also presents new opportunities for growth within the industry.
Rising Demand for Personalized Medicine
The Biologic Excipient Market is witnessing a notable shift towards personalized medicine, which is reshaping the landscape of drug development. As healthcare providers increasingly focus on tailoring treatments to individual patient needs, the demand for biologics that utilize specific excipients is likely to grow. This trend is supported by the fact that personalized therapies often require unique formulations, which depend heavily on the choice of excipients. In 2025, the market for personalized medicine is anticipated to exceed 100 billion USD, further driving the need for specialized biologic excipients. Consequently, manufacturers are compelled to innovate and develop excipients that can accommodate the complexities of personalized therapies, thereby enhancing their market position.
Advancements in Formulation Technologies
The Biologic Excipient Market is significantly influenced by advancements in formulation technologies. Innovations such as nanotechnology and microencapsulation are enabling the development of more effective biologic products. These technologies enhance the delivery and stability of biologics, which is crucial for their therapeutic effectiveness. As a result, the demand for biologic excipients that can support these advanced formulations is expected to increase. In 2025, the market for advanced drug delivery systems is projected to reach around 50 billion USD, indicating a strong correlation between technological advancements and the growth of the biologic excipient market. This dynamic environment encourages manufacturers to invest in research and development to create excipients that meet the evolving needs of the industry.