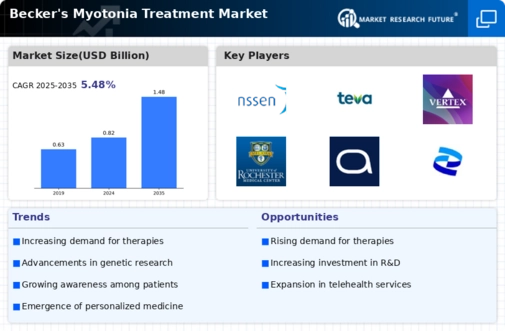
Growing Awareness and Advocacy
Growing awareness and advocacy for Becker's Myotonia are crucial drivers for the Becker's Myotonia Treatment Market. Patient advocacy groups and healthcare organizations are actively working to educate the public and healthcare professionals about this condition. Increased awareness leads to earlier diagnosis and treatment, which is essential for managing symptoms effectively. Moreover, advocacy efforts are fostering collaboration between stakeholders, including researchers, clinicians, and pharmaceutical companies, to enhance treatment options. This collective push for recognition and support is likely to result in a more favorable environment for the Becker's Myotonia Treatment Market, ultimately benefiting patients and healthcare providers alike.
Advancements in Genetic Research
Advancements in genetic research are significantly influencing the Becker's Myotonia Treatment Market. The identification of specific genetic mutations associated with Becker's Myotonia has opened new avenues for targeted therapies. As researchers delve deeper into the genetic underpinnings of this condition, the potential for developing personalized treatment regimens increases. This shift towards precision medicine is expected to enhance treatment efficacy and patient outcomes. Furthermore, the integration of genetic testing into clinical practice may facilitate earlier diagnosis and intervention, thereby expanding the market for Becker's Myotonia treatments. The ongoing research efforts in this domain suggest a promising future for innovative therapeutic options.
Rising Prevalence of Becker's Myotonia
The increasing prevalence of Becker's Myotonia is a pivotal driver for the Becker's Myotonia Treatment Market. As awareness of this condition grows, more individuals are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that Becker's Myotonia affects approximately 1 in 100,000 individuals, indicating a substantial patient population that requires management. This rising incidence not only amplifies the need for innovative therapies but also encourages pharmaceutical companies to invest in research and development. Consequently, the Becker's Myotonia Treatment Market is likely to witness significant growth as healthcare providers seek to address the needs of this expanding demographic.
Regulatory Support for Treatment Development
Regulatory support for treatment development is a significant factor influencing the Becker's Myotonia Treatment Market. Regulatory agencies are increasingly streamlining the approval processes for therapies targeting rare diseases, including Becker's Myotonia. This supportive environment encourages pharmaceutical companies to invest in research and development, knowing that there is a pathway to market for their innovations. Additionally, incentives such as orphan drug status can enhance the commercial viability of new treatments. As a result, the Becker's Myotonia Treatment Market is likely to experience accelerated growth, driven by the favorable regulatory landscape that promotes the development of effective therapies.
Increased Investment in Rare Disease Research
The Becker's Myotonia Treatment Market is benefiting from increased investment in rare disease research. Governments and private organizations are recognizing the need for effective treatments for rare conditions, including Becker's Myotonia. This influx of funding is likely to accelerate the development of novel therapies and improve access to existing treatments. In recent years, initiatives aimed at fostering innovation in rare disease research have gained momentum, resulting in a more robust pipeline of potential therapies. As a result, the Becker's Myotonia Treatment Market is poised for growth, driven by the commitment to address the unmet medical needs of patients suffering from this condition.