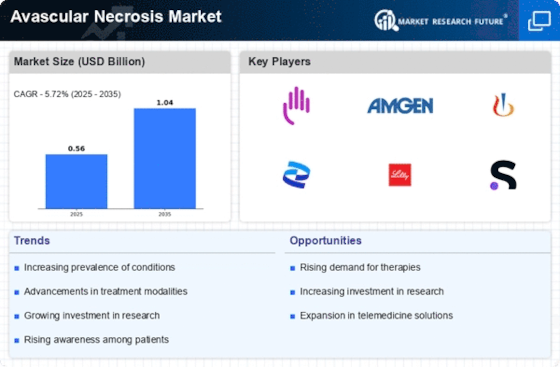
Top Industry Leaders in the Avascular Necrosis Market
 Latest Avascular Necrosis Companies Update
Latest Avascular Necrosis Companies Update
-
March 2023: An ellipsoid anatomic head, completely convertible stemmed total shoulder arthroplasty system was given 510(k) approval by the U.S. Food and Drug Administration. Indications for use of the shoulder system in anatomic or reverse arthroplasty are described in the FDA summary paper. If a reverse complete shoulder conversion is necessary, the humeral stem can be left in place and the articulating surfaces switched. Osteoarthritis (OA), avascular necrosis (AVN), rheumatoid arthritis (RA), post-traumatic arthritic diseases, and functional deformity repair are all included. The device must be "substantially equivalent" to a "predicate" device in order to receive 510(k) clearance. Compared to the AltiVateTM Shoulder System, this one is nearly identical in terms of "intended use and indications, materials, size ranges, and design intent."
-
May 2022: A phase II study of Regrow Biosciences' flagship product OSSGROW, an orphan medicine that helps efficiently treat Osteonecrosis, was approved by the US Food and medicine Administration in May 2022, the company reported. The medicine is designed for those who have Avascular Necrosis (also known as Osteonecrosis). According to the company's news release, Regrow Biosciences has taken a major step toward achieving a $5 billion worldwide market monopoly as a result of this achievement. While osteonecrosis is common in Asia, it is quite uncommon in Western countries. OSSGROW ® has received Orphan Drug Designation (ODD) from both the US Food and Drug Administration and the European Medicines Agency. After completing Phase 3 clinical studies in India, the business disclosed this information.
List of Avascular Necrosis Key companies in the market
- Novartis AG
- Pfizer Inc
- Teva Pharmaceutical Industries Ltd
- Mylan N.V
- Zimmer Biomet
- Merck & Co. Inc
- Enzo Biochem Inc
- Atnahs
- Bone Therapeutics SA
- Vericel Corporation.